COMPUTATIONAL MECHANISTIC STUDY OF THE DIELS–ALDER REACTION BETWEEN THE <i>N</i>-SUBSTITUTED EXOCYCLIC OXAZOLIDIN-2-ONE DIENE AND SYMMETRIC BIS-CHALCONE
Keywords:
benzoxazolone, chalcone, DFT, Diels–Alder cycloaddition, regioselectivity, stereoselectivityAbstract
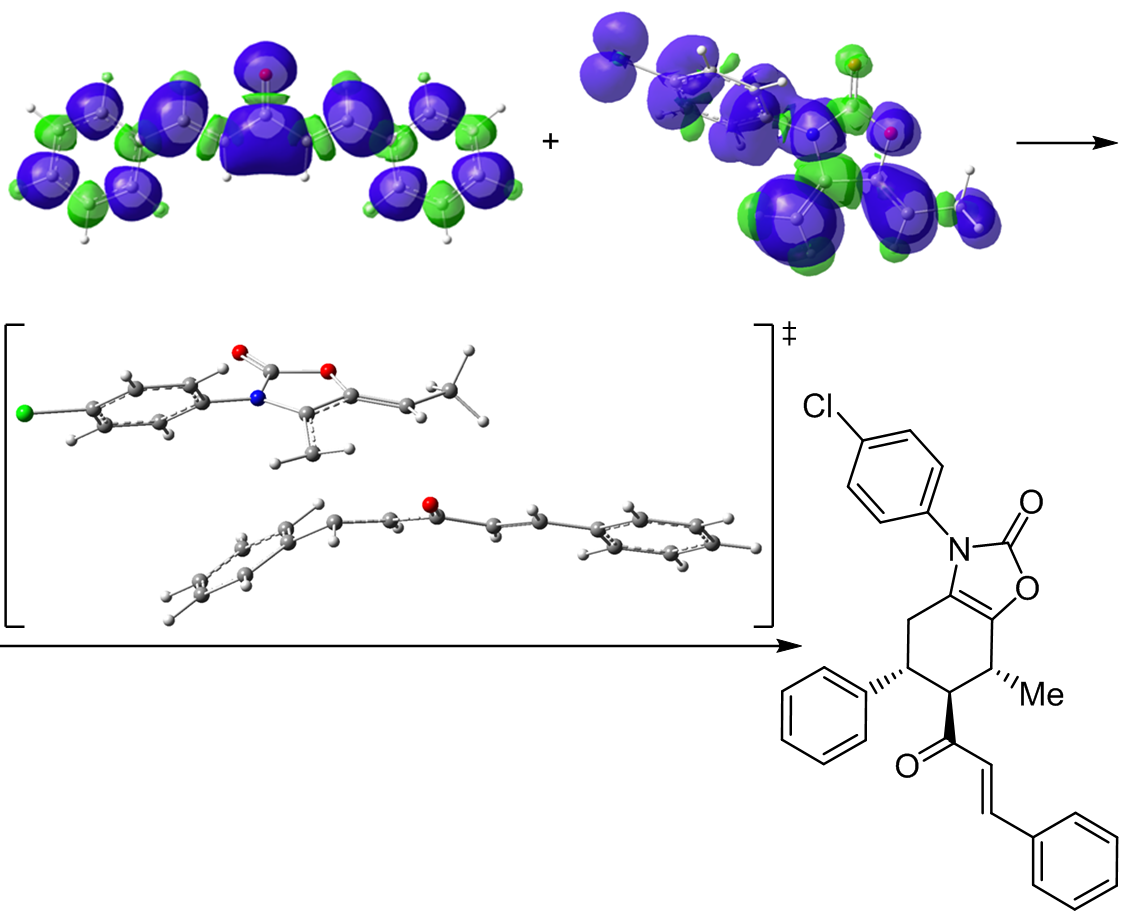
A computational study of the [4+2] cycloaddition reaction of N-substituted exocyclic oxazolidin-2-one diene with symmetric bischalcone was carried out at the B3LYP/6-311G(d,p) computational level in order to unravel the origin of the stereoselectivity experimentally observed. Analysis of the conceptual density functional theory (CDFT) reactivity indices indicates that the diene is a strong nucleophile while bis-chalcone is a good electrophile, accounting for a polar process. The Parr functions indices explain well the experimentally obtained ortho regioselectivity. Analysis of the energy profiles of the possible reactive pathways in gas phase and in solution of toluene indicate for exo stereoselective and ortho regioselective reaction, in excellent agreement with the experimental findings. Analysis of the transition states structures indicates for highly asynchronous mechanism. Bond evolution theory analysis of the most favorable pathway reveal for two-stage one-step molecular mechanism of the investigated reaction.