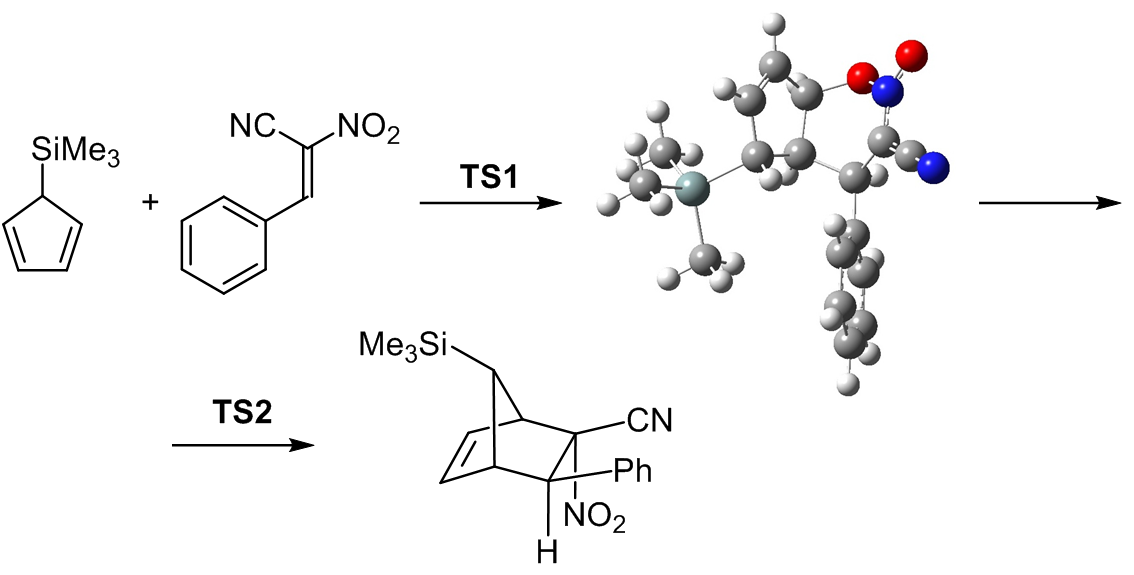
BICYCLIC 1,2-OXAZINE 2-OXIDES AS THE DISCRETE INTERMEDIATES IN THE REACTIONS BETWEEN REGIOISOMERIC TRIMETYLSILYLCYCLOPENTADIENES AND (2<i>E</i>)-2-NITRO-3-PHENYLPROP-2-ENENITRILE
Keywords:
heterocycles, nitroalkenes, 1,2-oxazine N-oxides, [4+2] cycloaddition, hetero-Diels–Alder reactionAbstract
The Diels–Alder reaction between regioisomeric trimethylsilylcyclopentadienes and (2E)-2-nitro-3-phenylprop-2-enenitrile leads initially to the formation of highly intriguing intermediates with a bicyclic 1,2-oxazine 2-oxide structure. In this study, we investigated the reaction mechanism using a combination of computational approaches. Conceptual density functional theory (CDFT) was applied to determine global and local reactivity indices of the reactants. Furthermore, the structures of the heterocyclic intermediates were analyzed using the electron localization function (ELF), providing insight into electron distribution and bonding patterns. Energy profiles of the reaction pathways were also computed to evaluate the thermodynamic and kinetic feasibility of the process.