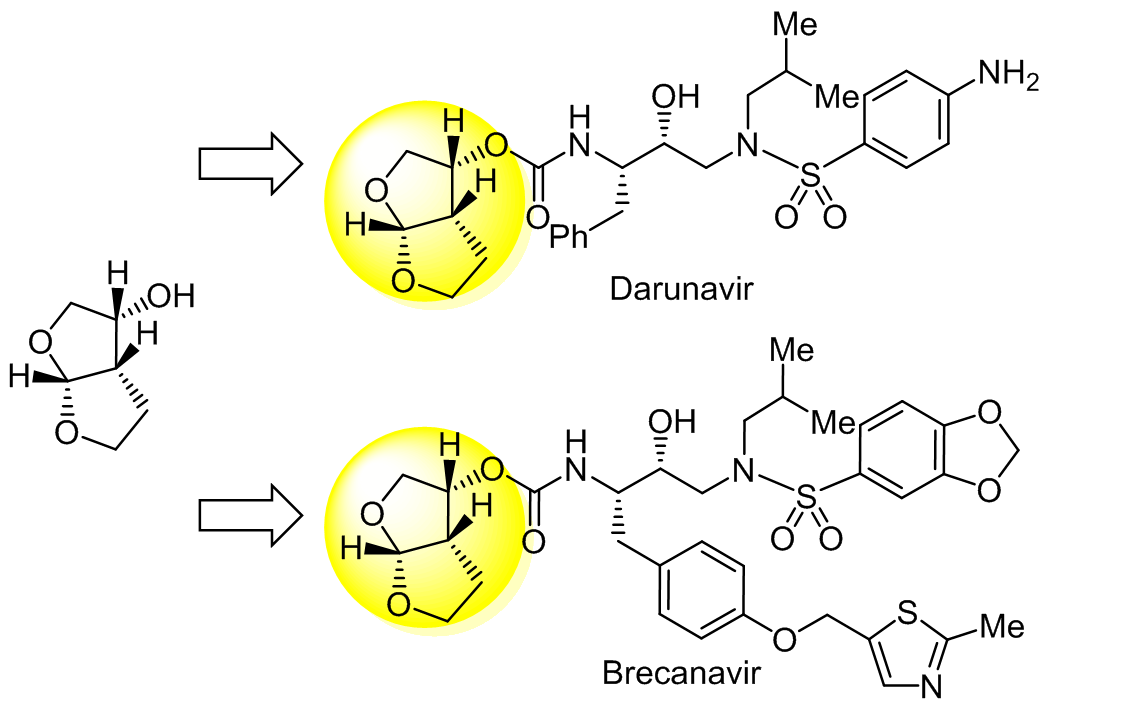
Advances in the synthesis of (3<i>R</i>,3a<i>S</i>,6a<i>R</i>)-hexahydrofuro[2,3-<i>b</i>]furan-3-ol, a key ligand of the HIV protease inhibitors
Keywords:
bis-THF alcohol, HIV protease inhibitors, asymmetric metal catalysis, chiral-substrate-induced reaction, enzyme-catalyzed resolutionAbstract
(3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol (bis-THF alcohol) is a key intermediate for the synthesis of HIV protease inhibitors with lower toxicity and better drug resistance. Due to its unique structure with three chiral centers and the high cost of this double-ring compound, chemists have paid much attention to the development of new synthetic routes. Based on the sources of the chiral carbon in bis-THF alcohol, this work systematically summarizes the existing synthetic processes using enzyme-catalyzed resolution, asymmetric metal catalysis, and chiral-substrate-induced reactions.
Downloads
Published
2024-09-25
Issue
Section
Review Articles
