SYNTHESIS AND <i>IN VITRO</i> ANTICANCER EVALUATION OF FUNCTIONALIZED 5-(4-PIPERAZIN-1-YL)-2-ARYLOXAZOLES AND 5-[(4-ARYLSULFONYL)PIPERAZIN-1-YL]-2-PHENYLOXAZOLES
Keywords:
2-aryloxazoles, 4-arylsulfonylpiperazines , oxazoles, sulfonamides, anticancer activity, heterocyclizationAbstract
This research focuses on the synthesis and in vitro anticancer evaluation of functionalized 2-aryl-5-(4-piperazin-1-yl)oxazoles and 5-[(4-arylsulfonyl)piperazin-1-yl]-2-phenyloxazoles. Oxazoles are a versatile class of compounds with diverse biological activities, making them
attractive targets in medicinal chemistry. We incorporated amino and sulfonamide functionalities into the oxazole scaffold, as they have shown potential for interacting with biological targets. The synthesis of target oxazole derivatives was accomplished using 2-aroylamino-
3,3-dichloroacrylonitriles as starting materials and employing efficient reaction conditions. The resulting compounds exhibited structural features that make them promising candidates for further chemical modifications and biological evaluations. Additionally, a series of
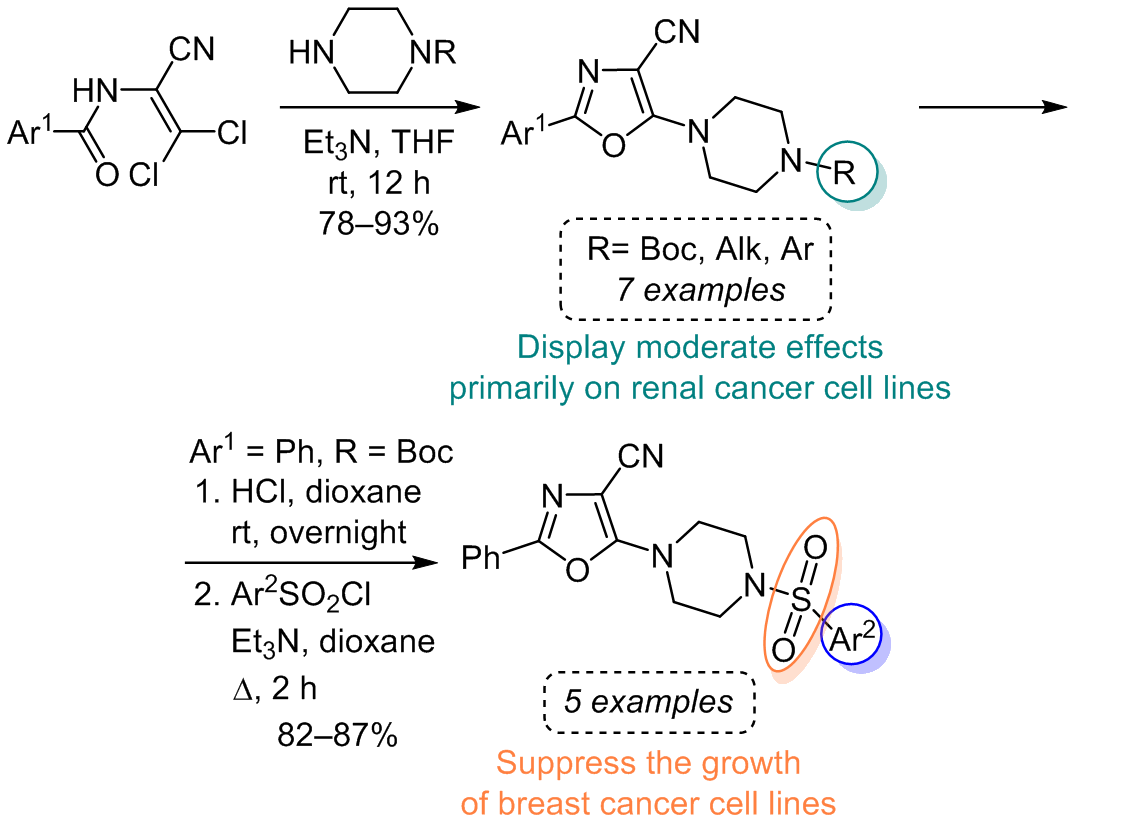
sulfonamides were synthesized from 5-(piperazin-1-yl)oxazole-4-carbonitrile hydrochloride, offering diverse bioactivity and versatile structural characteristics. However, no potent inhibitors of malignant cell growth were identified among the tested compounds. Nevertheless, we categorized the investigated substances into two distinct groups based on their activity profile. Group A, comprising
sulfonamides, displayed pronounced anticancer activity against breast cancer and melanoma cell lines. On the other hand, group B, the 2-aryl-5-(4-R-piperazin-1-yl)oxazole-4-carbonitriles, exhibited a moderate effect primarily on renal cancer cell lines. These findings provide valuable insights for further structural modifications in the quest for more potent anticancer agents.