A CASCADE REACTION OF 4-AMINO-SUBSTITUTED 6-HYDRAZINYL-1,3,5-TRIAZIN-2(1<i>H</i>)-ONES WITH TRIETHYL ORTHOACETATE
Keywords:
hydrazinyl-1,3,5-triazin-2(1H)-ones, [1,2,4]triazolo[1,3,5]triazines, [1,2,4]triazolo[4,3-a][1,3,5]triazin-2-ium-5-olates, triethyl orthoacetate, N- and O-alkylation with triethyl orthoacetate, [4,3-a]- and [1,5-a]-isomers, cascade reaction, Dimroth-type rearrangementAbstract
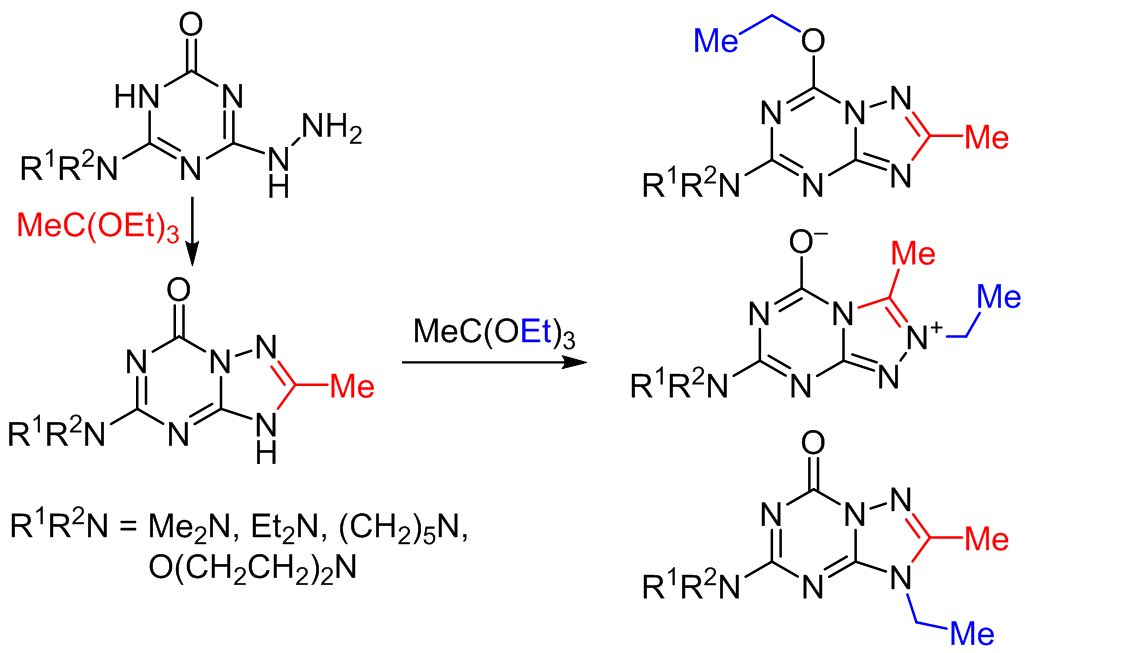
The reaction of 4-amino-substituted 6-hydrazinyl-1,3,5-triazin-2(1H)-ones with triethyl orthoacetate proceeded as a cascade reaction. The initially formed 7-amino-substituted 3-methyl[1,2,4]triazolo[4,3-a][1,3,5]triazin-5(1H)-ones underwent a Dimroth-type rearrangement with the formation of 5-amino-substituted 2-methyl[1,2,4]triazolo[1,5-a][1,3,5]triazin-7(3H)-ones. The latter underwent alkylation at three positions: at the exocyclic oxygen atom with the formation of 7-ethoxy-2-methyl[1,2,4]triazolo[1,5-a][1,3,5]triazines, at the N-3 nitrogen atom with the formation of 3-ethyl-2-methyl[1,2,4]triazolo[1,5-a][1,3,5]triazin-7(3H)-ones, and at the N-1 nitrogen atom to form 1-ethyl-2-methyl[1,2,4]triazolo[1,5-a][1,3,5]triazin-7(3H)-ones. For the latter compound, a retro-Dimroth-type rearrangement occurred with the formation of betaine-type 7-amino-substituted 2-ethyl-3-methyl[1,2,4]triazolo[4,3-a][1,3,5]triazin-2ium-5-olates. A possible mechanism for the alkylation of 5-amino-substituted 2-methyl[1,2,4]triazolo[1,5-a][1,3,5]triazin-7(3H)-ones with ortho esters was proposed.