A SYNTHESIS OF NOVEL 5-METHYLSULFANYLAZOLO[1,5-<i>a</i>]PYRIMIDIN-7(4<i>H</i>)-ONES AND INVESTIGATION OF THEIR CHEMICAL AND CYTOTOXIC PROPERTIES
Keywords:
azolo[1,5-a]pyrimidines, Meldrum's acid, heterocyclization, electrophilic substitution, antitumor activityAbstract
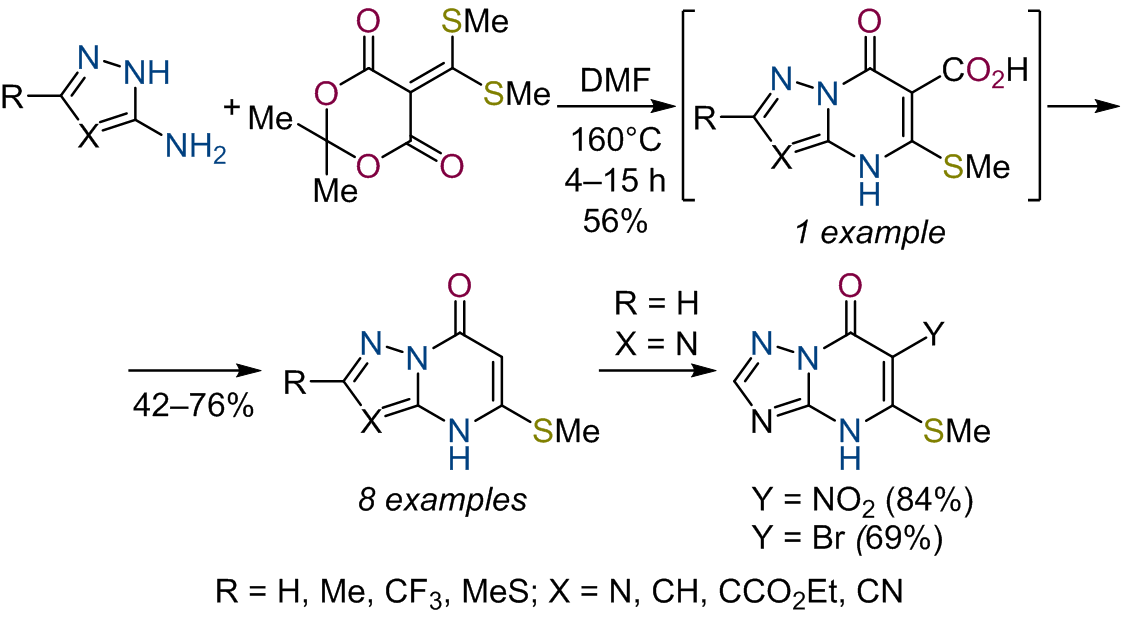
A method for the synthesis of novel 5-methylsulfanylazolo[1,5-a]pyrimidin-7(4H)-ones by heterocyclization of 3-aminoazoles and
5-[bis(methylsulfanyl)methylidene]-2,2-dimethyl-1,3-dioxane-4,6-diones was developed. 5-Methylsulfanyl-7-oxo-4,7-dihydroazolo-
[1,5-a]pyrimidine-6-carboxylic acid was isolated during optimization of the process, which allowed us to establish the sequence of
transformations of this heterocyclization reaction. The reactivity of the resulting 5-methylsulfanylazolo[1,5-a]pyrimidin-7(4H)-ones in
classical electrophilic substitution reactions was studied. The cytotoxic effect of these compounds toward A549, HepG2, and RD tumor
cell lines as well as normal HEK-293 cells was assessed.
Downloads
Additional Files
Published
2024-03-26
Issue
Section
Original Papers
