AN OXIDATIVE CLEAVAGE OF ARENE-CONDENSED 4<i>H</i>-PYRANS <i>VIA</i> THE GROB–WHARTON FRAGMENTATION
Keywords:
m-chloroperoxybenzoic acid, 12,13-dihydrobenzo[f]indeno[1,2-b]chromenes, 12,14-dihydro-13H-dibenzo[a,h]xanthenes, ketolactones, oxecine-2,7-diones, oxonine-2,6-diones, Grob–Wharton fragmentationAbstract
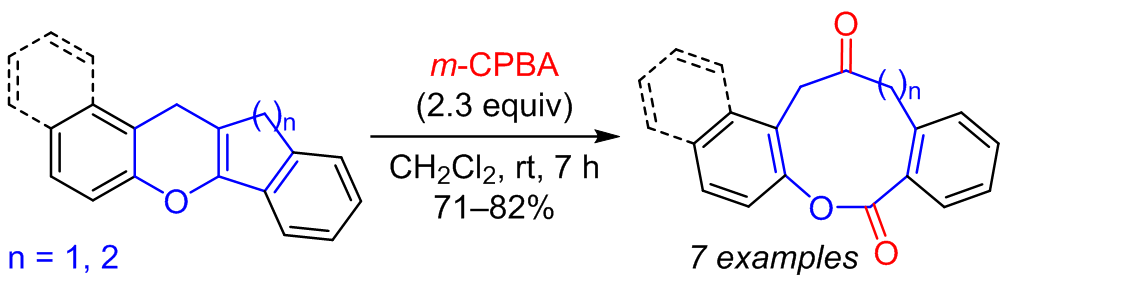
When benzannulated dihydroindenochromene and dihydroxanthene derivatives were treated with m-chloroperoxybenzoic acid, oxidative cleavage of the pyran C=C bond and the formation of condensed ketolactones, the derivatives of oxonine-2,6-dione and oxecine-2,7-dione, took place. The reaction proceeded via epoxidation of the pyran double bond followed by the opening of the epoxide and Grob–Wharton fragmentation.
Downloads
Additional Files
Published
2024-05-28
Issue
Section
Original Papers
