Synthesis of ursane-derived regioisomeric 2-amino-1,3,4-oxadiazoles and 3-thioxo-1,2,4-triazoles
Keywords:
acylsemicarbazide, acylthiosemicarbazide, 2-amino-1,3,4-oxadiazole, norursane, 3-thioxo-1,2,4-triazole, ursaneAbstract
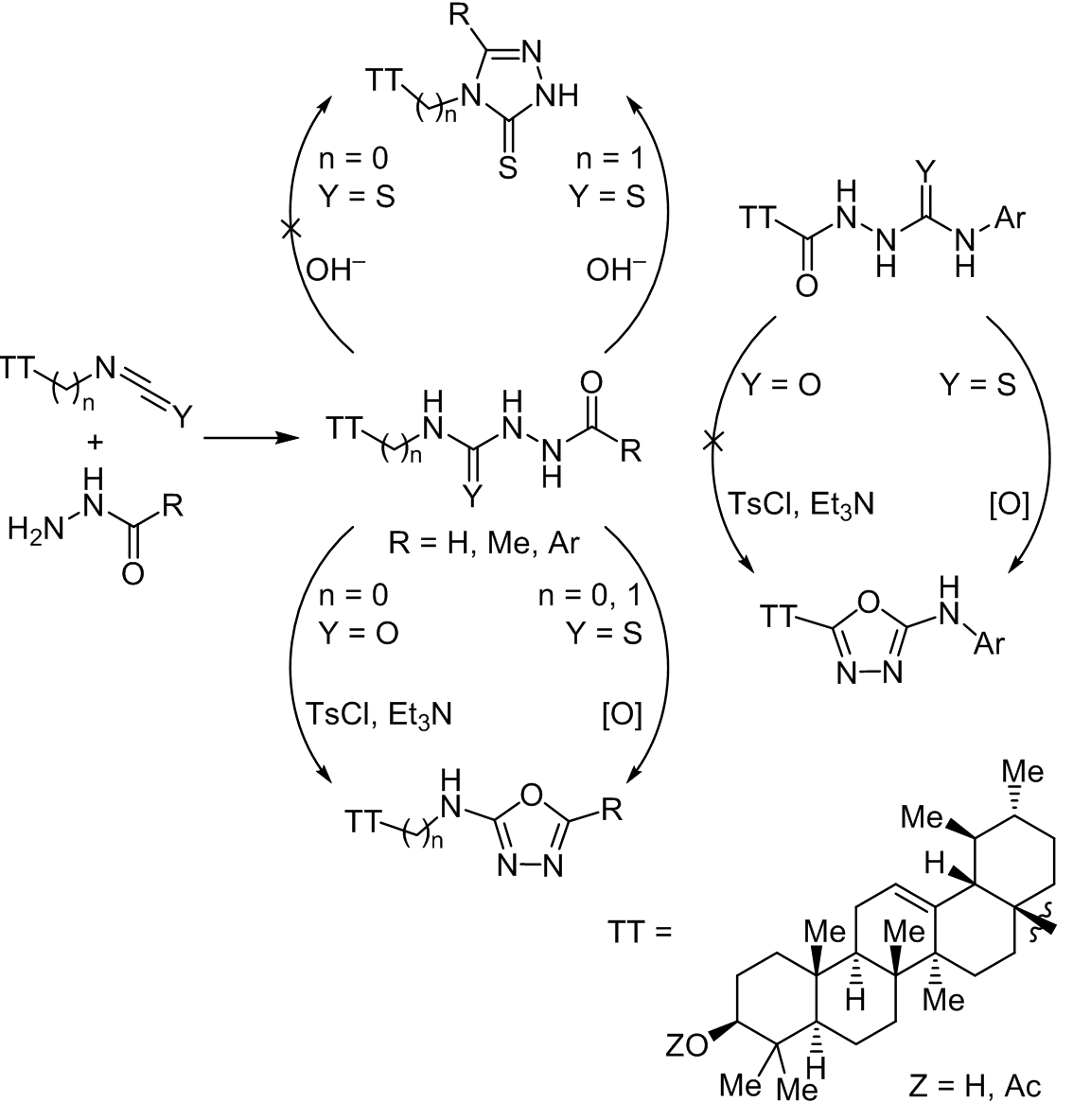
In the present work, novel ursane-derived 2-amino-1,3,4-oxadiazoles and 3-thioxo-1,2,4-triazoles were prepared. Coupling aliphatic or aromatic hydrazides with terpenoids containing NCO or NCS groups at C-17 atom of norursane or a NCS group at C-28 atom of the ursane backbone led to substituted acyl(thio)semicarbazides. 4-Terpenyl-3-thioxo-1,2,4-triazoles were obtained from formyl- and acetylthiosemicarbazides containing a thiourea group at C-28 atom of the ursane core (with 72 and 82% yields, respectively). The cyclodehydration of acylsemicarbazides with an urea fragment at C-17 atom of norursane with TsCl–Et3N afforded 2-(terpenylamino)-1,3,4-oxadiazoles (yield 75–84%), including hybrids with strong electron acceptor substituents unavailable through norursane-derived sulfur intermediates (norursane-17-isothiocyanates did not react with poorly nucleophilic hydrazides). However, the synthesis of regioisomeric 2-(arylamino)-5-terpenyl-1,3,4-oxadiazoles from norursane acylsemicarbazide derived from ursolic acid hydrazide was not feasible because of the steric constraint of intramolecular attack. The oxidative cyclization of acylthiosemicarbazides is the method of choice for the preparation of norursane-type 2-(arylamino)-5-terpenyl-1,3,4-oxadiazoles.