DESIGN, SYNTHESIS, AND FUNGICIDAL ACTIVITY EVALUATION OF 2-METHYL-5-PHENYLTHIAZOLE-4-CARBOXAMIDES BEARING MORPHOLINE, THIOMORPHOLINE, OR THIOMORPHOLINE 1,1-DIOXIDE MOIETY
Keywords:
morpholine derivatives, thiazole derivatives, thiomorpholine derivatives, fungicidal activity, structural optimization, structure– activity relationshipAbstract
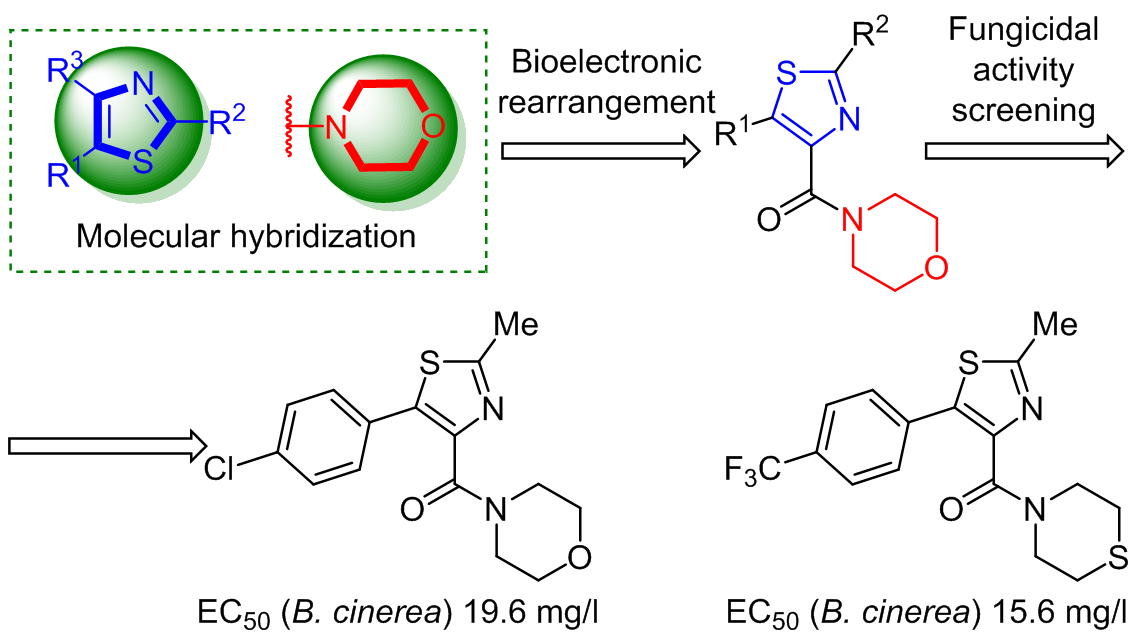
A series of 2-methyl-5-phenylthiazole derivatives were designed, synthesized, and characterized by 1H, 13C NMR spectra and HRMS. Subsequently, their antifungal activity was evaluated, and the bioassay results showed that almost all title compounds possess potential fungicidal activity against B. cinerea. [5-(4-Chlorophenyl)-2-methylthiazol-4-yl](morpholin-4-yl)methanone and {2-methyl-5-[4-(trifluoromethyl)phenyl]thiazol-4-yl}(thiomorpholin-4-yl)methanone displayed EC50 values of 19.6 mg/l and 15.6 mg/l respectively, against B. cinerea, showing higher potency than that of hymexazol.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
