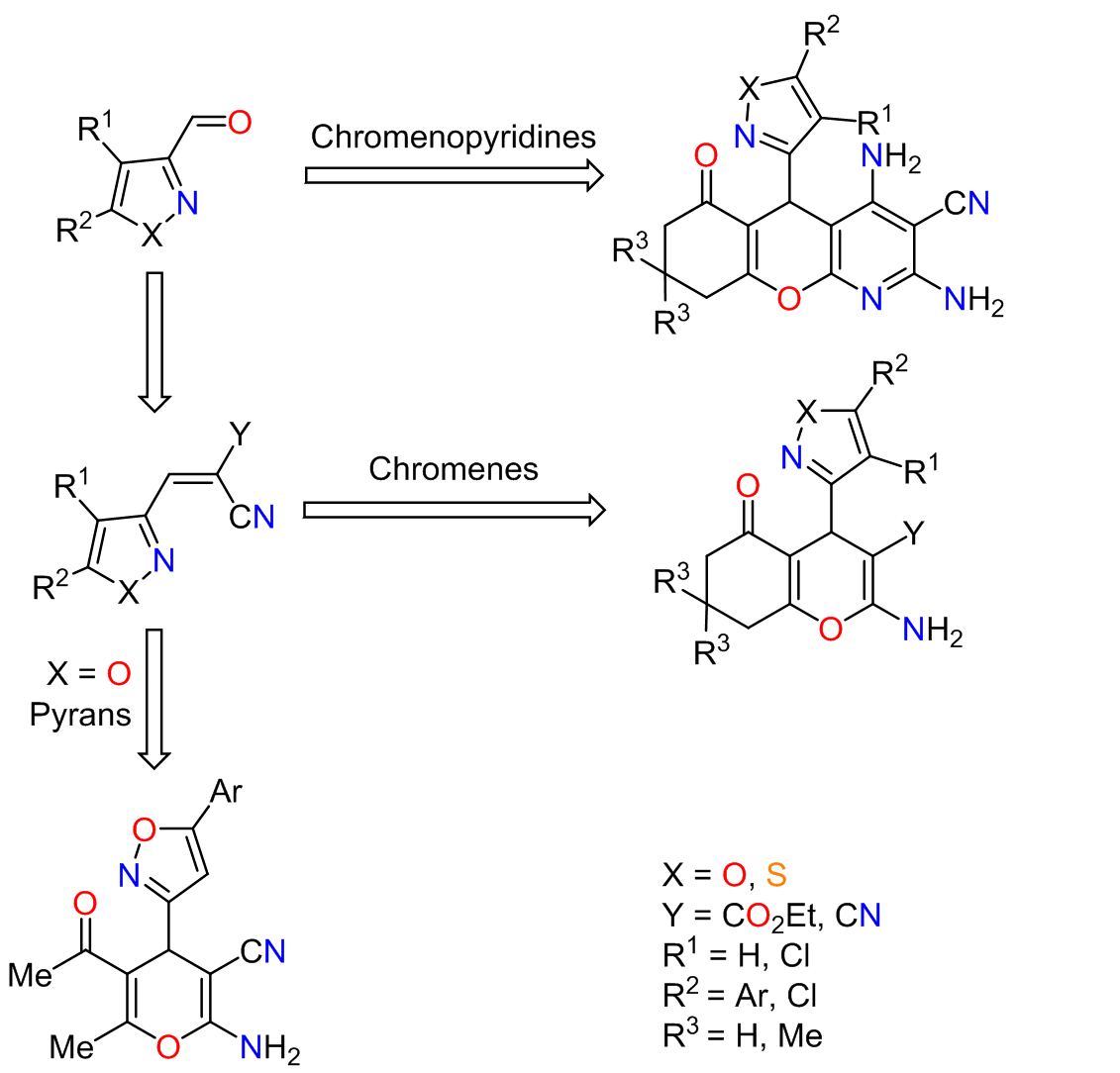
Polysubstituted pyrans, chromenes, and chromenopyridines with isoxazole or isothiazole moiety: synthesis, structure, and antitumor activity
Keywords:
aldehydes, chromenes, chromenopyridines, isothiazoles, pyrans, Knoevenagel reactionAbstract
Polysubstituted derivatives of pyrans, chromenes, and chromeno[2,3-b]pyridines incorporating a 4,5-dichloroisothiazole or 5-arylisoxazole-3-carbaldehyde fragment in the molecule were synthesized by successive and one-step three-component reactions of 4,5dichloroisothiazole-3-carbaldehyde and 5-arylisoxazole-3-carbaldehyde with various 1,3-diketones, malononitrile or its dimer. The structure of the target products was proven by X-ray structural analysis. Notably, it was found that the planes of the azole and chromene rings in the molecules of (1,2-azol-3-yl)chromenes are almost perpendicular. Intrinsic antitumor activity and synergistic enhancement of the activity in combination with known cytostatics used in the therapy of oncological diseases were found for some of the synthesized compounds.