A ONE-POT SYNTHESIS OF 3-NITRO-2<i>H</i>-THIOPYRANS AND THEIR SELECTIVE REDUCTION TO 3-NITRO-3,4-DIHYDRO-2<i>H</i>-THIOPYRANS
Keywords:
synthesis of 2H-thiopyrans, Lawesson's reagent, β-nitrostyrenes, 2-phenylbenzimidazoline, one-pot synthesis, reduction of 2H-thiopyransAbstract
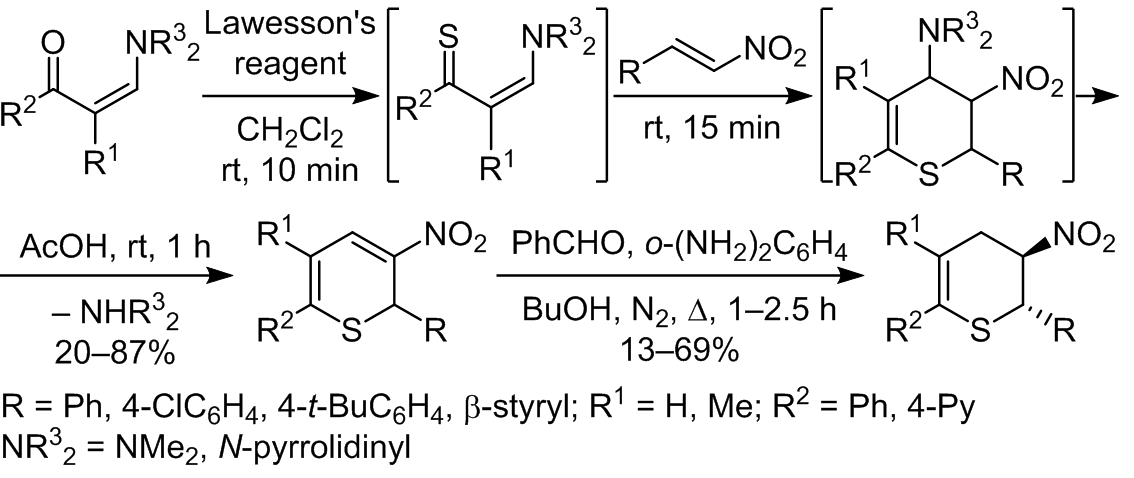
We developed a one-pot method for the synthesis of non-annealed 3-nitro-2H-thiopyrans based on enamine-3-ones. Reduction of the resulting 3-nitro-2H-thiopyrans with the benzaldehyde/ortho-phenylenediamine system in butanol leads to 3-nitro-3,4-dihydro-2H-thiopyrans.
Downloads
Additional Files
Published
2024-08-01
Issue
Section
Original Papers
