AN INTRAMOLECULAR DIELS–ALDER REACTION IN THE SYNTHESIS OF <i>N</i>-AROYL-3a,6-EPOXYISOINDOLE-2-CARBOTHIOAMIDES
Keywords:
allylamine, epoxyisoindole, furfurylamine, isothiocyanate, thiourea, IMDAF reaction, intramolecular [4+2] cycloadditionAbstract
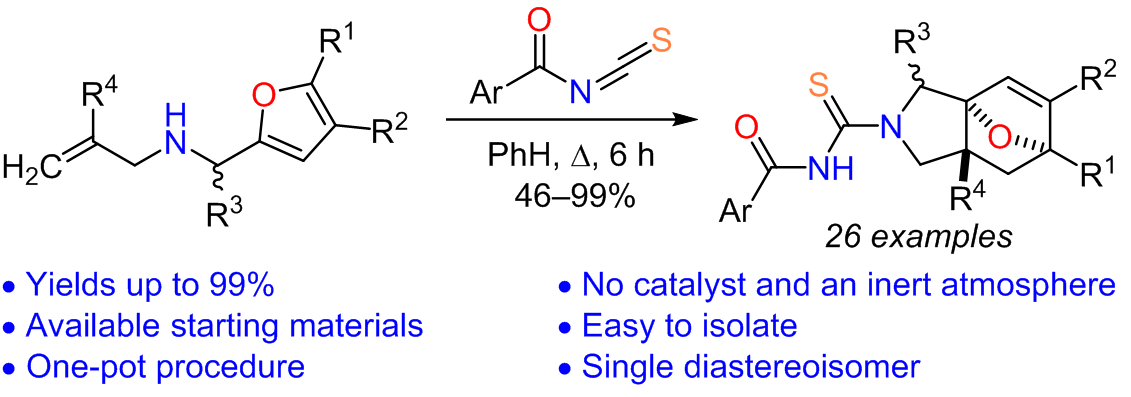
The reaction of allyl(furfuryl)amines with aroyl isothiocyanates was studied. The reaction proceeded via an initial nucleophilic addition of the allylamine nitrogen atom to isothiocyanates and a subsequent spontaneous intramolecular Diels–Alder reaction involving the furan ring of intermediate N-allyl-N-furfurylthioureas with the formation of a single diastereomer of 3a,6-epoxyisoindoles.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
