PIDA-MEDIATED C–N COUPLING CYCLIZATION FOR THE SYNTHESIS OF 3-ARYL-1<i>H</i>-INDAZOLES
Keywords:
hypervalent iodine reagent, indazole, vinylhydrazone, C–N coupling cyclization, oxidative aromatizationAbstract
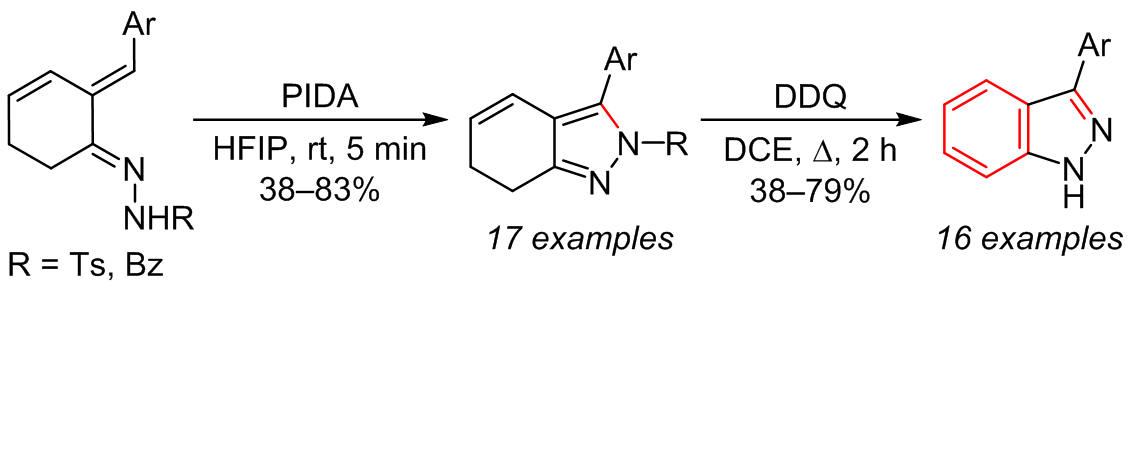
Synthesis of 6,7-dihydro-2H-indazoles by iodobenzene diacetate mediated intramolecular C–N coupling cyclization of acylhydrazones derived from (E)-2-methylidenecyclohex-3-enones was reported for the first time. Oxidative aromatization of these dihydroindazole intermediates with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone followed by in situ deprotection of the indazole nitrogen produced a series of 3-aryl-1H-indazoles in moderate to high yields. The cyclization method has the advantages of mild reaction conditions, broad substrate scope, and operational simplicity, which provides a novel synthetic route toward indazole derivatives and enriches the application of hypervalent iodine reagents in the synthesis of N-heterocyclic compounds.