SYNTHESIS OF POLYANILINE, POLYPYRROLE, AND POLY(ANILINE-<i>co</i>-PYRROLE) IN DEEP EUTECTIC SOLVENT: A COMPARATIVE EXPERIMENTAL AND COMPUTATIONAL INVESTIGATION OF THEIR STRUCTURAL, SPECTRAL, THERMAL, AND MORPHOLOGICAL CHARACTERISTICS
Keywords:
Polyaniline, Polypyrrole, Copolymerisation, Band gap, DFT calculations, Electrical and optical propertiesAbstract
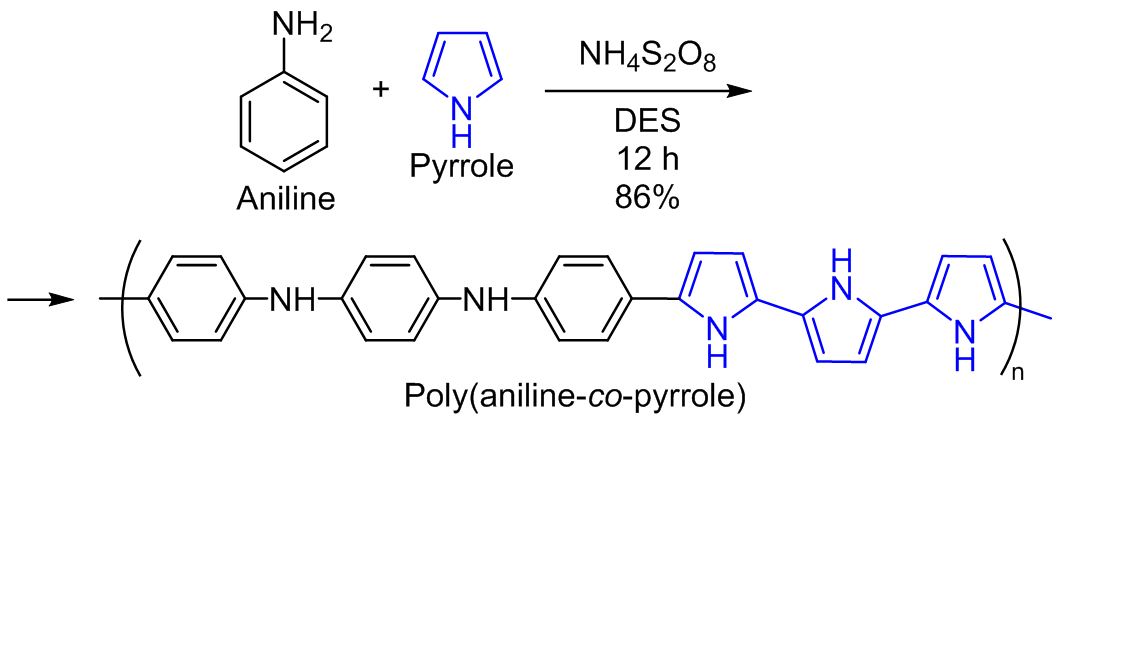
In this investigation, deep eutectic solvent based on propionic acid and choline chloride was used to prepare polyaniline, polypyrrole, and poly(aniline-co-pyrrole) samples using chemical oxidation and polymerization. Quantum mechanical calculations were conducted using time-dependent density functional theory to determine the electronic, spectroscopic properties and structure of homopolymers and the copolymer which were compared with the respective experimental data. Furthermore, the synthesized materials were characterized using Fourier transform IR spectroscopy, scanning electron microscopy (SEM), UV-visible spectroscopy, and thermogravimetric analysis. IR and UV-visible spectra confirmed copolymerization, and SEM revealed that the copolymer had a mixed morphology uniting features of both polyaniline and polypyrrole. Furthermore, the copolymer composite was more thermally stable than both homopolymeric materials. The quantum-chemical investigation revealed significant variations in the electron affinities and ionization energies of three polymers. The copolymer has the highest HOMO electronic density, indicating its superior electron-donating capability, while also having the highest LUMO density, suggesting a strong electron-accepting potential. Further, it also exhibits the smallest energy gap (2.384 eV), implying its high chemical reactivity, low stability, and high degree of polarization. The findings suggest that a larger energy gap correlates with greater molecular hardness and lower electronic softness, with polyaniline exemplifying these properties.