THE SYNTHESIS AND ANTIPROLIFERATIVE ACTIVITY OF ISATIN-7-SULFONAMIDES
Keywords:
formamidine protecting group, isatin, sulfonamides, antiproliferative activity, Sandmeyer reactionAbstract
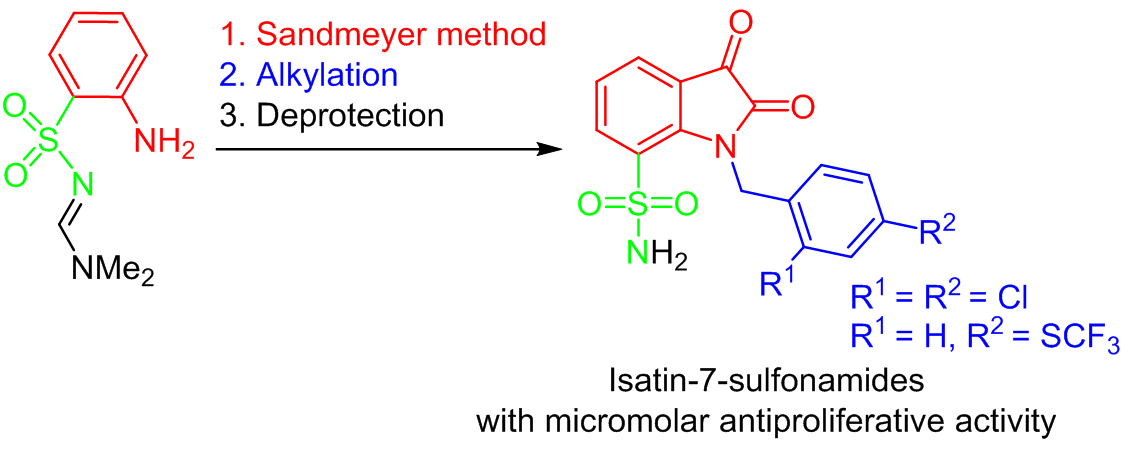
Varying the position of the pharmacophore group in a heterocyclic ring can have a significant effect on the biological activity of compounds. By means of the formamidine protecting group and the Sandmeyer reaction, a route of synthesis of previously undescribed isatin-7-sulfonamides was developed. The synthesized 1-substituted isatin-7-sulfonamides inhibited the growth of hematological and solid tumor cells in low micromolar concentrations. The obtained data indicate the promise of 1-substituted isatin-7-sulfonamides as a new pharmacophore for the development of compounds with antitumor activity.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
