EFFICIENT CONSTRUCTION OF SPIROCYCLIC AZAOXINDOLES <i>VIA</i> DOUBLE MICHAEL ADDITION REACTION USING PHASE-TRANSFER CATALYSIS
Keywords:
7-azaoxindole, dienones, spirocyclic azaoxindoles, double Michael addition, phase-transfer catalysisAbstract
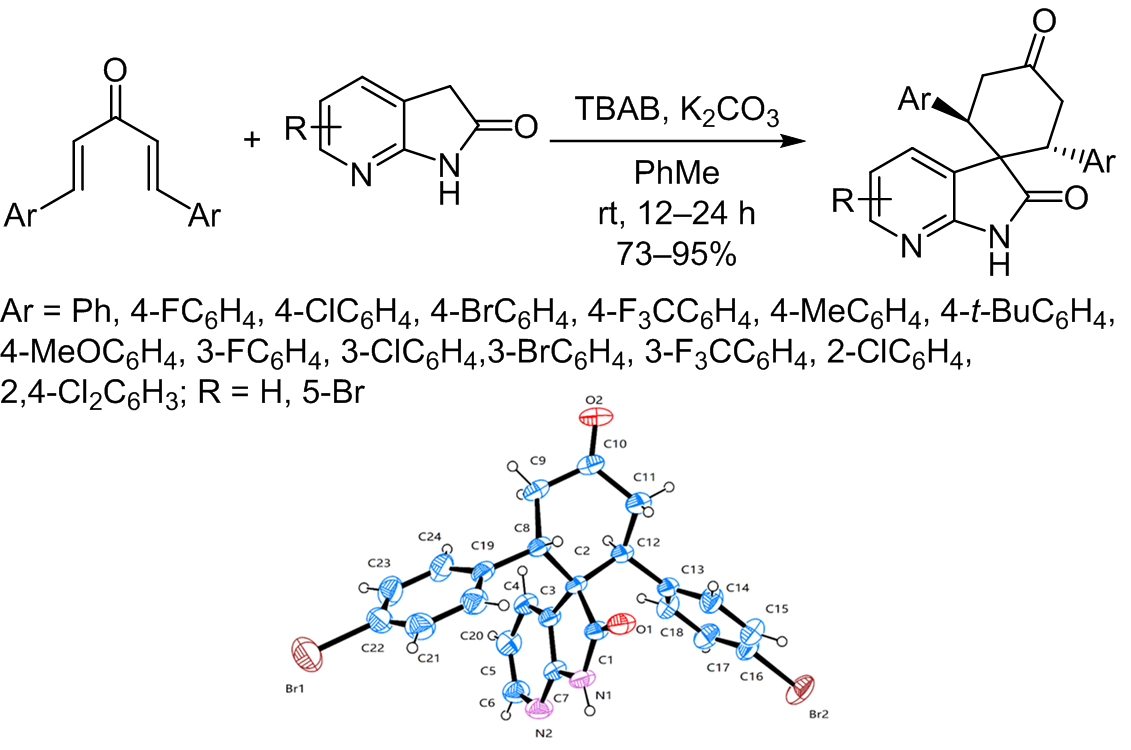
The double Michael addition reaction is a highly effective approach for constructing spirocyclic compounds, which frequently occur in nature and exhibit biological activity. In this study, the double Michael addition reaction of 7-azaoxindole with dienones was explored using tetrabutylammonium bromide as a catalyst. The target spirocyclic azaoxindoles were obtained in moderate to high yields.
Downloads
Additional Files
Published
2025-05-28
Issue
Section
Original Papers
