A [4+2] CYCLOADDITION OF PUSH-PULL STYRENES TO 1,2-NAPHTHOQUINONE 1-METHIDES: A SYNTHESIS OF 2-ARYL-2,3-DIHYDRO-1<i>H</i>-BENZO[<i>f</i>]CHROMENES
Keywords:
2-aryl-2,3-dihydro-1H-benzo[f]chromenes, isoflavans, 2-naphthol Mannich bases, o-quinone methides, Cope reaction, Diels–Alder reaction, push-pull styrenesAbstract
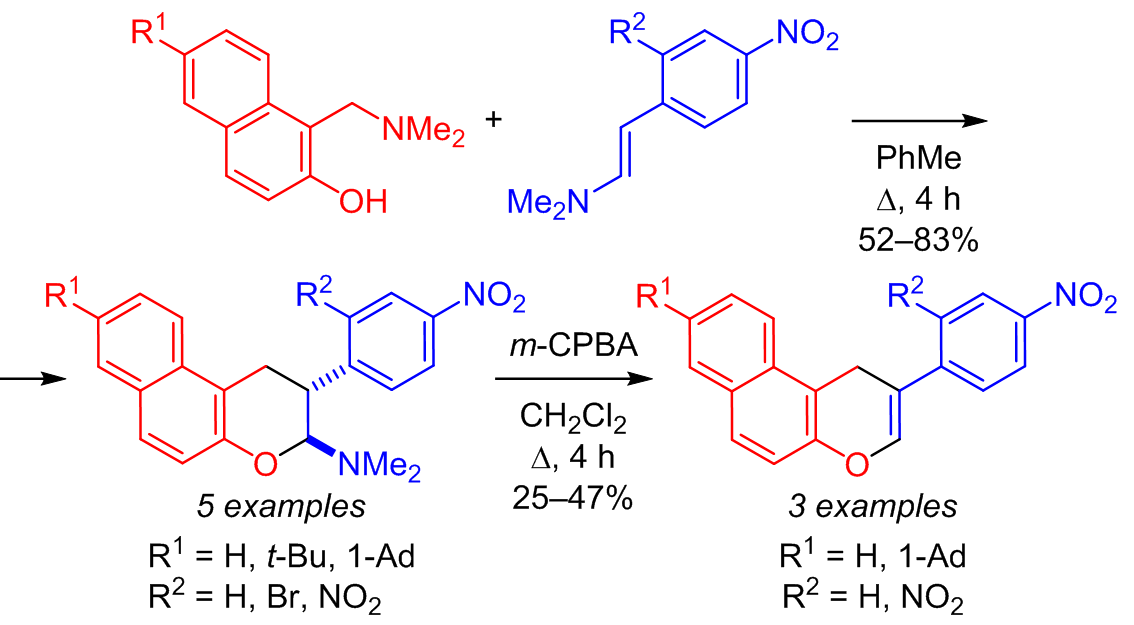
A regioselective and trans-diastereoselective method for the preparation of 2-aryl-2,3-dihydro-1H-benzo[f]chromenes based on 2-naphthol Mannich bases as precursors of 1,2-naphthoquinone 1-methides and highly polarized β-(N,N-dimethylamino)styrene was developed. The resulting cycloadducts were transformed into cyclic acetals and hemiacetals as well as introduced into the Cope reaction leading to 2-aryl-1H-benzo[f]chromenes.