SOME PROPERTIES OF 1-HYDROXY-1<i>H</i>-IMIDAZOLE-2-CARBOXYLIC ACID ESTERS
Keywords:
amides, esters of 1-hydroxy-1H-imidazole-2-carboxylic acids, hydrazides, alkylation, bromination, nitration, reductionAbstract
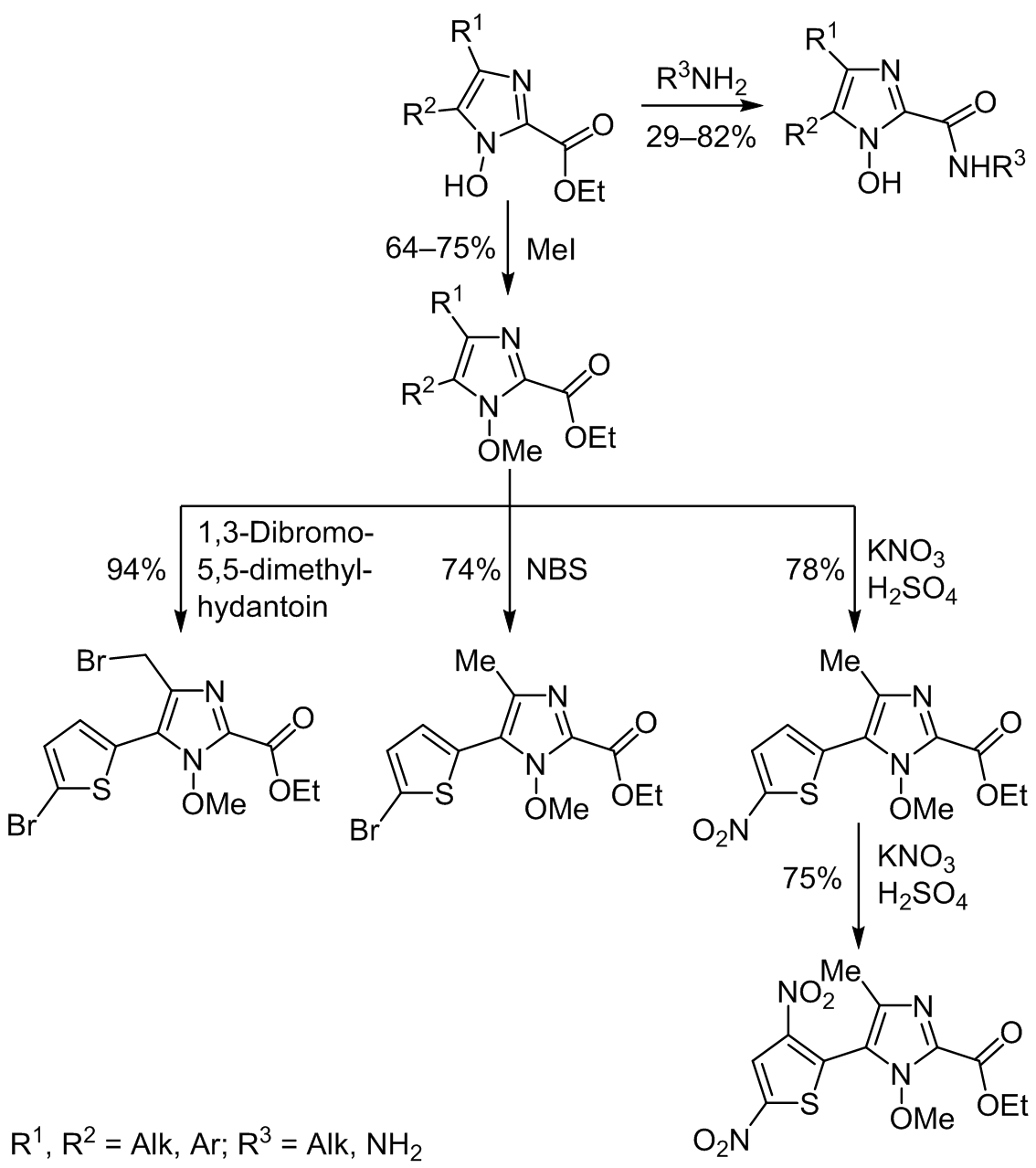
The corresponding amides and hydrazides were obtained by the reaction of 1-hydroxy-1H-imidazole-2-carboxylates with amines and hydrazine. In the reaction of an ester of 1-methoxy-4-methyl-5-(thiophen-2-yl)-1H-imidazole-2-carboxylic acid with NBS, bromination occurred at the thiophene ring, whereas in the reaction with 1,3-dibromo-5,5-dimethylhydantoin it took place at both the methyl group and the thiophene ring; its nitration led to mononitro- or dinitrothiophene derivatives. The reaction of 1-hydroxy-1H-imidazole-2-carboxylates with chloroacetone in Me2CO in the presence of Et3N resulted in the formation of reduced compounds.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
