THE CYCLOADDITION REACTION OF BENZOTHIAZOLIUM YLIDES WITH α-CYANOCINNAMAMIDES: THE SYNTHESIS OF STRUCTURAL ANALOGS OF INHIBITORS OF HIV-1 POST-INTEGRATIONAL REPAIR
Keywords:
azomethine ylides, benzothiazoles, 1,3-dipolar cycloaddition, HIV-1 inhibitors, stereoselectivityAbstract
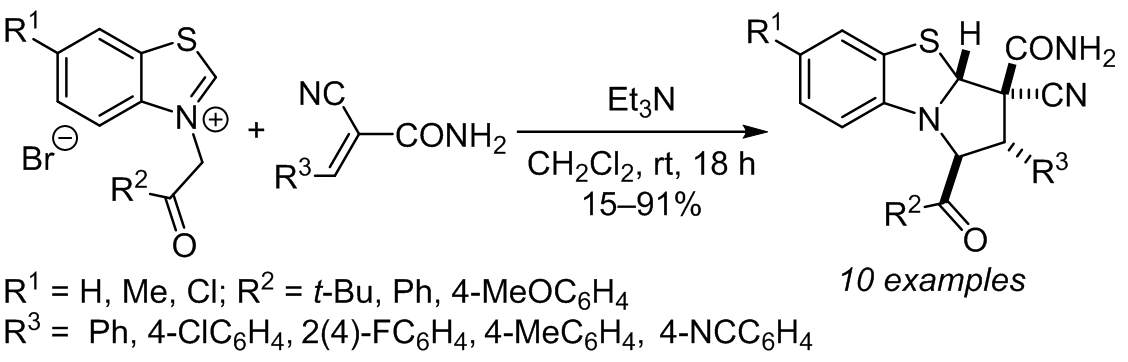
Benzothiazolium ylides generated in situ from benzothiazolium salts by the action of triethylamine reacted stereoselectively with α-cyanocinnamamides to form 1,2,3,3a-tetrahydropyrrolo[2,1-b][1,3]benzothiazoles in 15–91% yields. The resulting compounds were significantly more stable than their analogs derived from quinolinium ylides (KuINin), inhibitors of HIV-1 post-integrational repair.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
