A NEW APPROACH TO THE PYRROLO[3,4-<i>d</i>]PYRIMIDINE SYSTEM <i>VIA</i> TANDEM STAUDINGER/AZA-WITTIG REACTION OF 5-ACYL-4-AZIDOMETHYL-3,4-DIHYDROPYRIMIDIN-2(1<i>H</i>)-ONES
Keywords:
N-[(2-azido-1-tosyl)alkyl]ureas, ureidoalkylation, Staudinger reaction, aza-Wittig reaction., 3,4-dihydropyrimidin-2(1H)-ones, pyrrolo[3,4-d]pyrimidinesAbstract
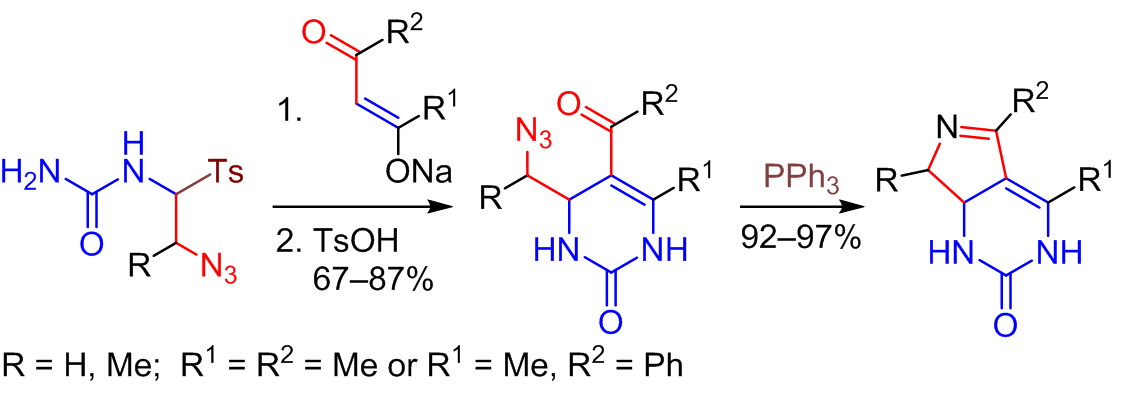
A novel access to pyrrolo[3,4-d]pyrimidine scaffold via tandem Staudinger/intramolecular aza-Wittig reaction of 5-acyl-4-(1-azidoalkyl)-3,4-dihydropyrimidin-2(1H)-ones promoted by PPh3 was developed. Synthesis of the starting pyrimidinones involved the reaction ofreadily available N-[(2-azido-1-tosyl)alkyl]ureas with Na enolates of benzoylacetone or acetylacetone followed by acid-catalyzed dehydration of the resulting products without their isolation.
Downloads
Published
2024-01-10
Issue
Section
Original Papers
