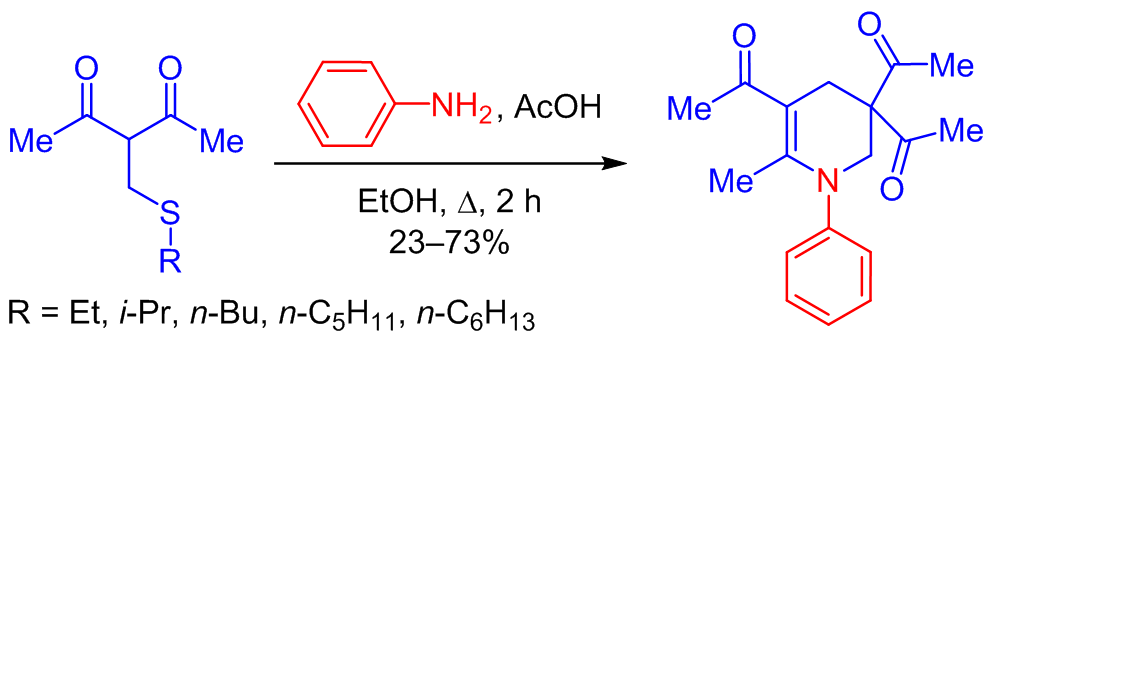
A SYNTHESIS OF TRIACETYL-SUBSTITUTED 1,2,3,4-TETRAHYDROPYRIDINE BY THE REACTION OF 3-[(ALKYLSULFANYL)METHYL]PENTANE-2,4-DIONES WITH ANILINE
Keywords:
3-[(alkylsulfanyl)methyl]pentane-2,4-dione, 1,2,3,4-tetrahydropyridine, aniline, cyclization, β-enaminone, [4+2] cycloadditionAbstract
The condensation of 3-[(alkylsulfanyl)methyl]pentane-2,4-diones with aniline in the presence of catalytic amounts of acetic acid led to the formation of 1,1',1''-(6-methyl-1-phenyl-1,2,3,4-tetrahydropyridine-3,3,5-triyl)triethanone. The reaction is presumably a tandem process, incorporating the formation of β-enaminone, elimination of the alkanethiol molecule from the β-enaminone and the starting pentane-2,4-dione, and a subsequent [4+2] cycloaddition of the resulting intermediates. The mechanism of the conversion may also involve intramolecular cyclization of the Michael addition products of 3-[(alkylsulfanyl)methyl]pentane-2,4-dione to 3-(imidoyl)but-3-en-2-one, formed from β-enaminones during the elimination of alkanethiols.