A SIMPLE METHOD FOR THE SYNTHESIS OF DIARYLAMINES CONTAINING A NITROSO GROUP IN THE ORTHO POSITION BASED ON THE S<sub>N</sub><sup>H</sup> ARYLAMINATION OF 5-NITROISOQUINOLINE
Keywords:
heterocycles, nitroso compound, metal-free C–N bond formation, oxidative SNH arylamination, redox process, SNH methodologyAbstract
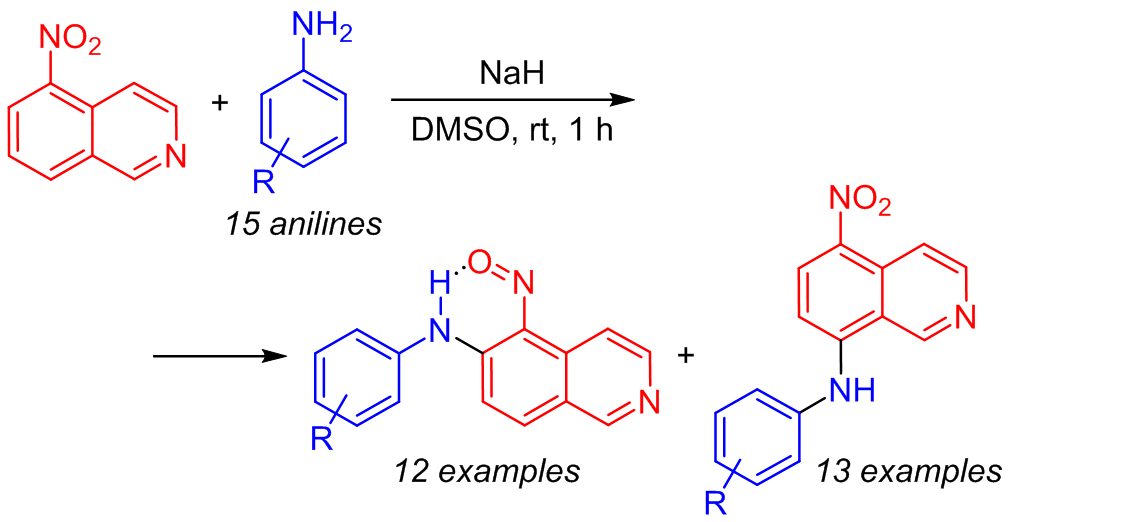
A simple and efficient arylamination reaction of 5-nitroisoquinoline based on the oxidative nucleophilic substitution of hydrogen was demonstrated under metal-catalyst-free conditions. This reaction can be used as a method for the synthesis of isoquinoline-based diarylamines containing a nitroso group in the ortho position, which are compounds with high synthetic potential. The oxidation of the latter leads to the formation of 6-arylamino-5-nitroisoquinoline N-oxides. The absence of the need to introduce leaving groups and a good overall yield are features of this reaction.
Downloads
Additional Files
Published
2024-05-28
Issue
Section
Original Papers
