CASCADE ASSEMBLING OF ALDEHYDES AND TWO MOLECULES OF DIMEDONE INTO 4<i>H</i>-SPIRO[1-BENZOFURAN-2,1'-CYCLOHEXANE]-2',4,6'-TRIONES UNDER COLUMN CHROMATOGRAPHY-FREE PROTOCOL AT ROOM TEMPERATURE
Keywords:
arylaldehydes, dimedone derivatives, spirodihydrofurans, cascade reaction, cyclization, tandem Knoevenagel–Michael reactionsAbstract
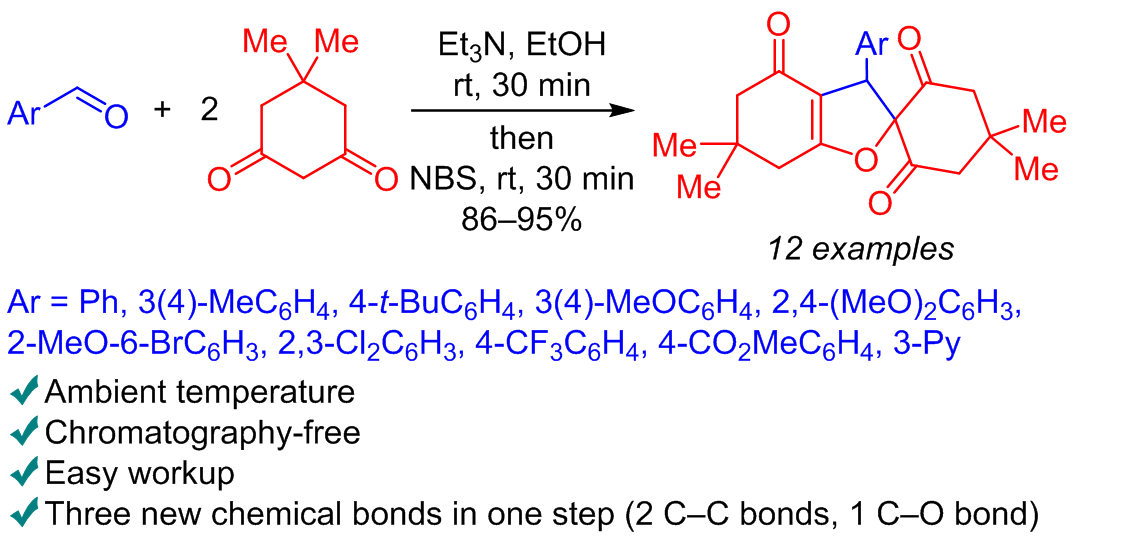
A new type of one-pot Knoevenagel–Michael reaction with the following NBS-induced cyclization was found: a direct one-pot transformation of aldehydes and two molecules of dimedone into substituted 4H-spiro[1-benzofuran-2,1'-cyclohexane]-2',4,6'-triones in 86–95% yields. This one-pot process is a very efficient and convenient way to access substituted 4H-spiro[1-benzofuran-2,1'-cyclohexane]-2',4,6'-triones – useful compounds for different biomedical applications with reasonable and nonexpensive starting materials. Mild and facile conditions of this chemical cascade one-pot process, as well as non-chromatographic isolation procedure lead to excellent substance yields.
Downloads
Additional Files
Published
2024-08-01
Issue
Section
Original Papers
