[3+3] Annulation of diethyl 2,3-dicyanofumarate and cyclic 1,3-dicarbonyl compounds: synthesis of fused diethyl 2-amino-4-cyano-4<i>H</i>-pyran-3,4-dicarboxylates
Keywords:
cyclic 1,3-dicarbonyl compounds, diethyl 2-amino-4-cyano-4H-pyran-3,4-dicarboxylates, diethyl 2,3-dicyanofumarate, Michael reaction, Thorpe–Ziegler cyclizationAbstract
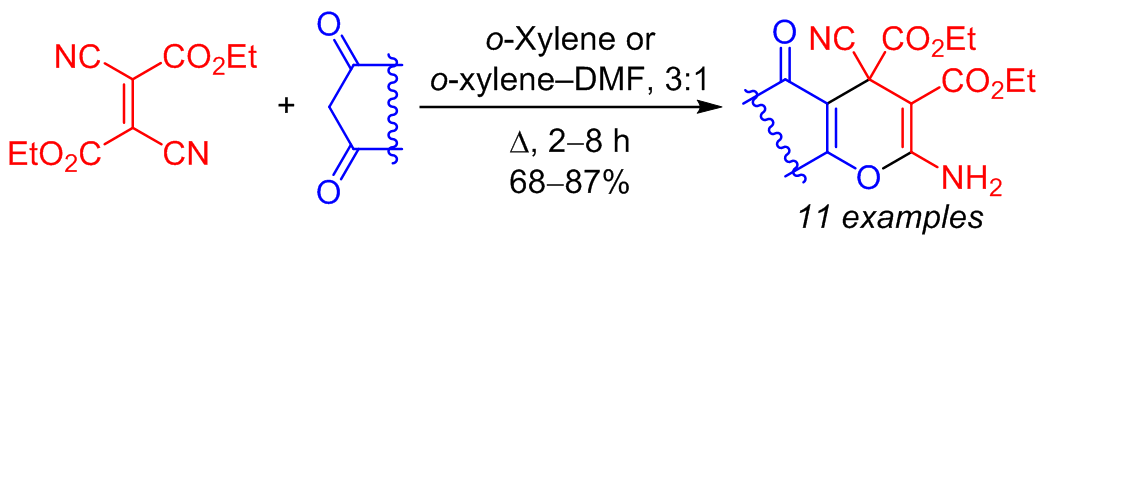
The reaction of diethyl 2,3-dicyanofumarate with carbo- and heterocyclic 1,3-dicarbonyl compounds resulted in a [3+3] annulation with the formation of condensed diethyl 2-amino-4-cyano-4H-pyran-3,4-dicarboxylates. The cascade transformation involved the Michael addition of the enol form of the CH acid to the double bond of diethyl 2,3-dicyanofumarate followed by the Thorpe–Ziegler cyclization.
Downloads
Published
2024-09-25
Issue
Section
Short Communications
