SELECTIVE REDUCTION OF 5,7-DINITRO-8-HYDROXYQUINOLINE AND SYNTHESIS OF 2-SUBSTITUTED 5-NITROOXAZOLO[4,5-<i>h</i>]QUINOLINES
Keywords:
nitroquinolines, 5,7-dinitro-8-oxyquinoline, 7-amino-5-nitroquinolin-8-ol, 5-nitrooxazolo[4,5-h]quinolines, Zinin reaction, chemical reduction, catalytic hydrogenation, cyclocondensationAbstract
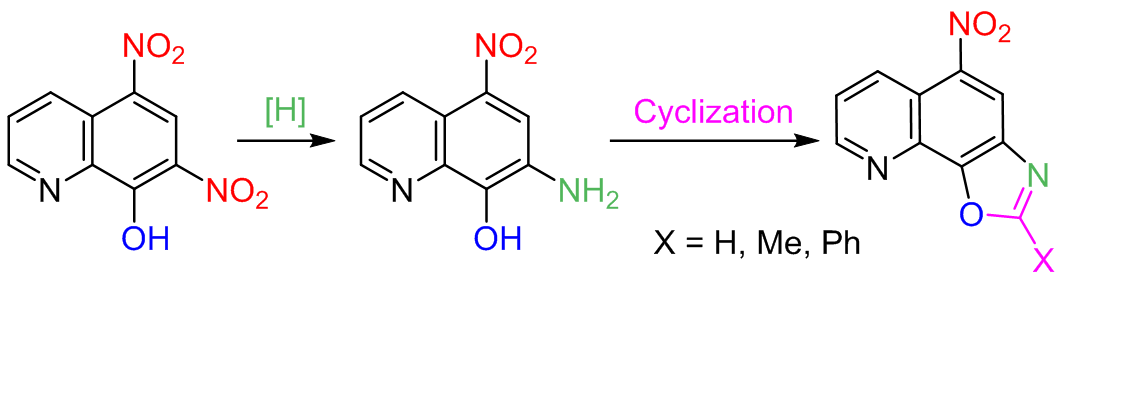
Chemoselective reduction of the ortho-nitro group in 5,7-dinitro-8-oxyquinoline was carried out by the action of Na2S in H2O and DMSO as well as by hydrogenation on 0.8% Pd/C. A number of new 2-substituted 5-nitrooxazolo[4,5-h]quinolines were obtained starting from the synthesized 7-amino-5-nitroquinolin-8-ol.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
