PROTON- AND IODINE-INDUCED CYCLIZATION OF 2-(PENT-4-EN-1-YL)QUINAZOLIN- 4(3<i>H</i>)-ONES: SYNTHESIS OF PYRROLO[2,1-<i>b</i>]QUINAZOLINONE AND PYRIDO[1,2-<i>a</i>]QUINAZOLINONE DERIVATIVES
Keywords:
2-(pent-4-en-1-yl)quinazolin-4(3H)-ones, pyrrolo(pyrido)quinazolinones, trifluoromethanesulfonic acid, annulations, iodocyclizationsAbstract
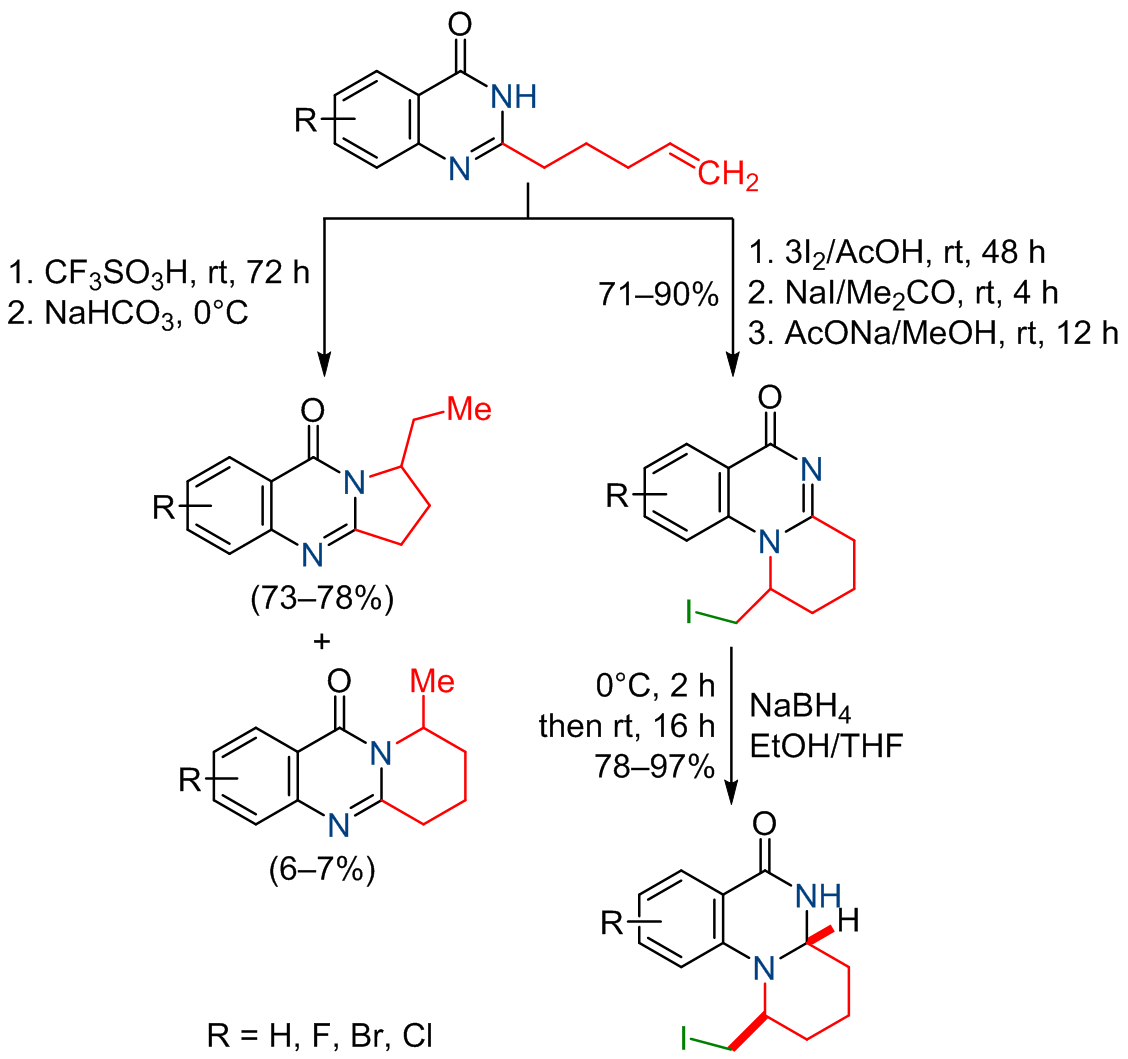
2-(Pent-4-en-1-yl)quinazolin-4(3H)-ones in CF3SO3H solution undergo preferential cyclization into 1-ethyl-2,3-dihydropyrrolo[2,1-b]quinazolin-9(1H)-ones via the intermediate rearrangement in the alkenyl moiety, while 9-methyl-6,7,8,9-tetrahydro-11H-pyrido[2,1-b]quinazolin-11-ones are minor products. Iodocyclization of 2-(pent-4-en-1-yl)quinazolin-4(3H)-ones proceeds according to the 6-exo-trig cyclization mode to form 1-(iodomethyl)-1,2,3,4-tetrahydro-6H-pyrido[1,2-a]quinazolin-6-ones, which are further converted to their hexahydro derivatives by the diastereoselective reduction with NaBH4.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
