SYNTHESIS AND ANTIPROLIFERATIVE ACTIVITY OF NEW OXAZOLE-STEROID GLYCOCONJUGATES
Ключевые слова:
oxazole, antiproliferative, anticancer, one-pot, steroidal N-glycoside, intramolecular oxidative cyclodesulfuration, natural products, carbohydrates, glycosteroidsАннотация
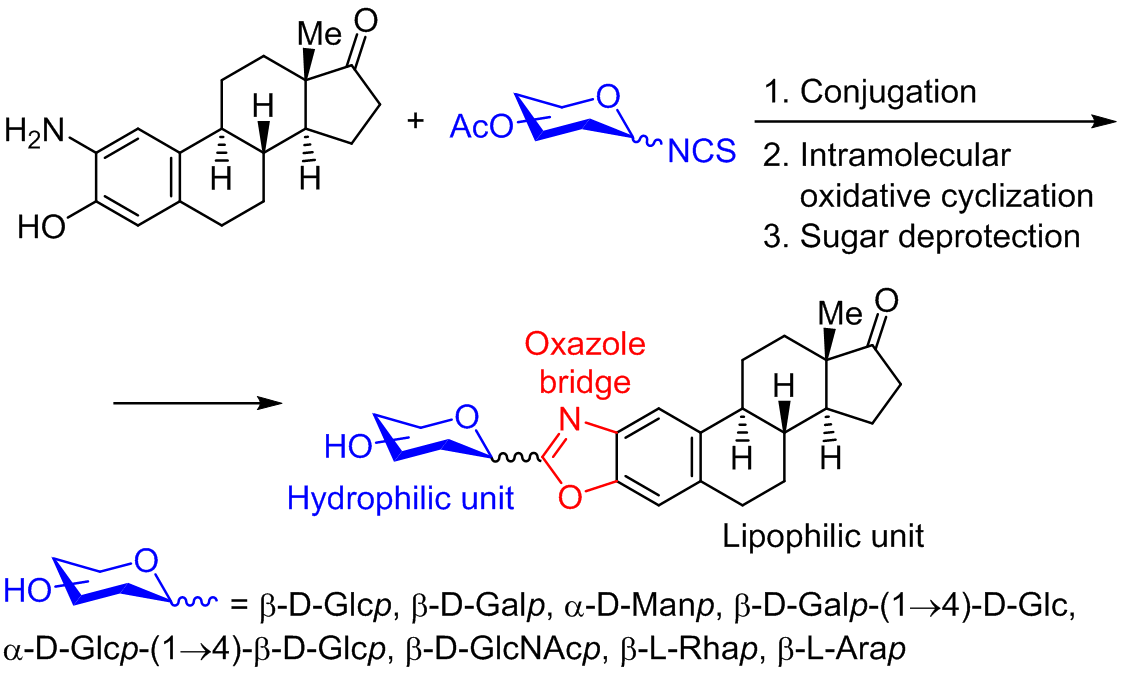
Conjugation of glycosyl isothiocyanates and steroidal amino alcohol derived from estrone, followed by an oxidative cyclodesulfuration reaction allowed the synthesis of a new class of bioactive molecules. This rapid and versatile protocol represents a useful approach toward the synthesis of a wide variety of novel 2-aminobenzoxazoles. The glycoconjugates (mono- and disaccharides) were successfully obtained in good to excellent yields. The acetyl-protected and deprotected compounds were screened for antiproliferative activity against six human cancer cell lines; a total of eight compounds showed mild to moderate activity. Remarkably, both derivatives bearing a mannose moiety exhibited GI50 values at the low μM range.