MULTICOMPONENT SYNTHESIS OF 1,5,6,7-TETRAHYDRO- 4<i>H</i>-INDOL-4-ONE DERIVATIVES
Ключевые слова:
arylglyoxals, multicomponent reactions, cyclohexane-1,3-diones, 1,5,6,7-tetrahydro-4H-indol-4-oneАннотация
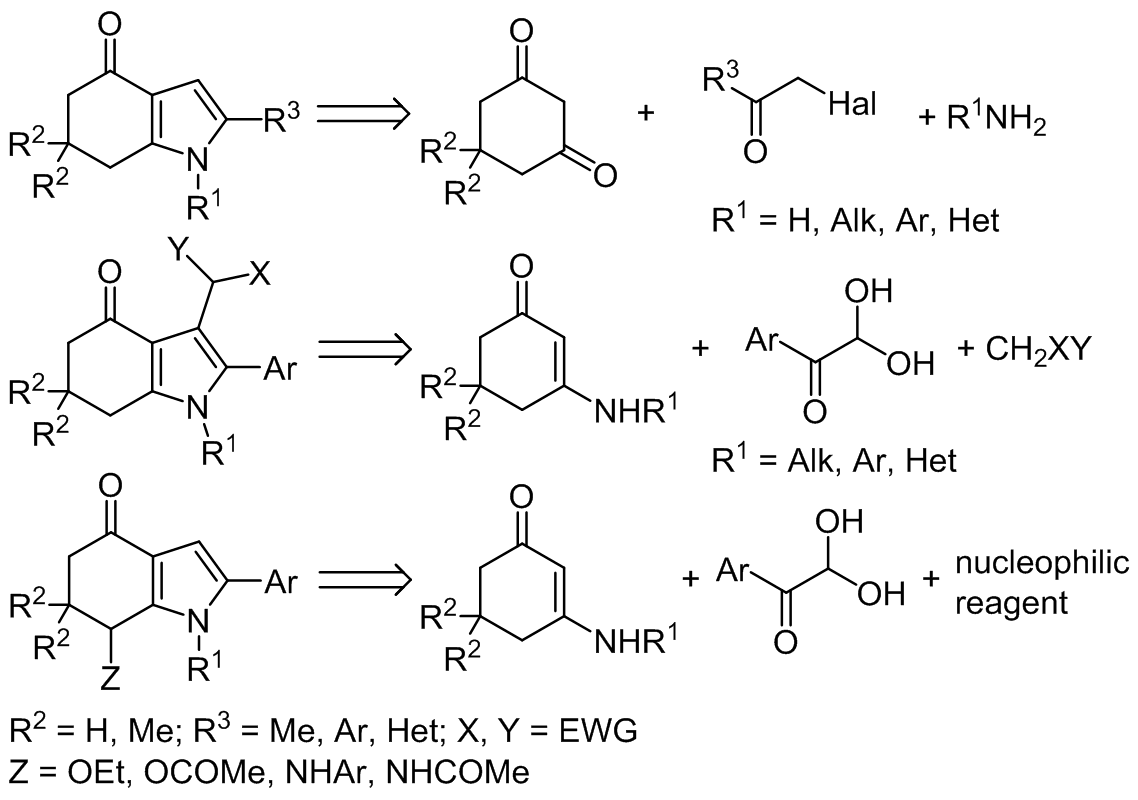
The data on multicomponent methods of tetrahydro-4H-indol-4-one synthesis published over the past ten years are summarized in this review. Three main synthetic approaches in the construction of such molecules are considered. Among them are: condensation of cyclohexane-
1,3-diones with α-halogenoketones and primary amines, three-component reaction of cyclic enaminoketones, arylglyoxals, and methylene active compounds, and condensation of cyclic enaminoketones and arylglyoxals in the presence of nucleophilic reagents (often solvents). The latter domino reaction runs under microwave irradiation and leads to the functionalization of position 7 of the indole ring system. The material is systematized according to the structure of the starting compounds.