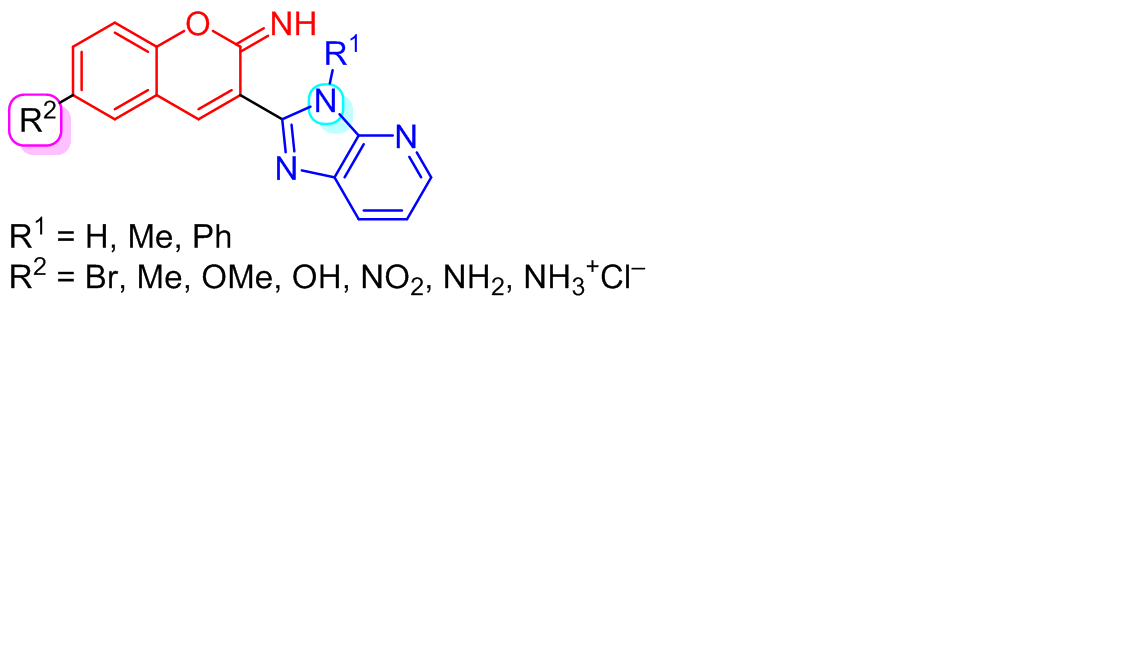
NOVEL IMINOCOUMARIN IMIDAZO[4,5-<i>b</i>]PYRIDINE DERIVATIVES: DESIGN, SYNTHESIS, AND BIOLOGICAL EVALUATION
Ключевые слова:
Carbonyl compounds, Microwave assisted synthesis, Imidazopyridines, Iminocoumarines, Biological activityАннотация
Herein, we present the design, synthesis, and biological activity of novel iminocoumarin imidazo[4,5-b]pyridine derivatives. The prepared compounds were designed to study the type of substituent in position 6 of the coumarin nucleus as well as the type of the substituent at the N atom of imidazo[4,5-b]pyridine core for their effect on the biological activity. Therefore, all compounds were tested for their antiproliferative action on several human cancer cell lines in vitro, for their antioxidative activity, antibacterial activity on several bacterial strains, and for their antiviral activity on several viruses. The results of the evaluation of biological activity revealed that the tested derivatives did not display significant biological activities. The majority of the tested compounds were not active at all, while some derivatives showed low activity in these assays. Therefore, we could conclude that the biological potential of 6-substituted iminocoumarin derivatives is very low – the substitution in position 6 of the coumarin nucleus, in comparison to 7-substituted iminocoumarins, strongly decreases biological activity.