THIAZOLO[5,4-<i>b</i>]INDOLE DERIVATIVES AS ADDITIVES TO CARDIOPLEGIC SOLUTIONS WITH INCREASED TIME OF PREVENTING HYPOTHERMIC ISCHEMIA
Ключевые слова:
antihypoxant, custodiol, indenotiasol, histological examination, dystrophic changes, myocardiumАннотация
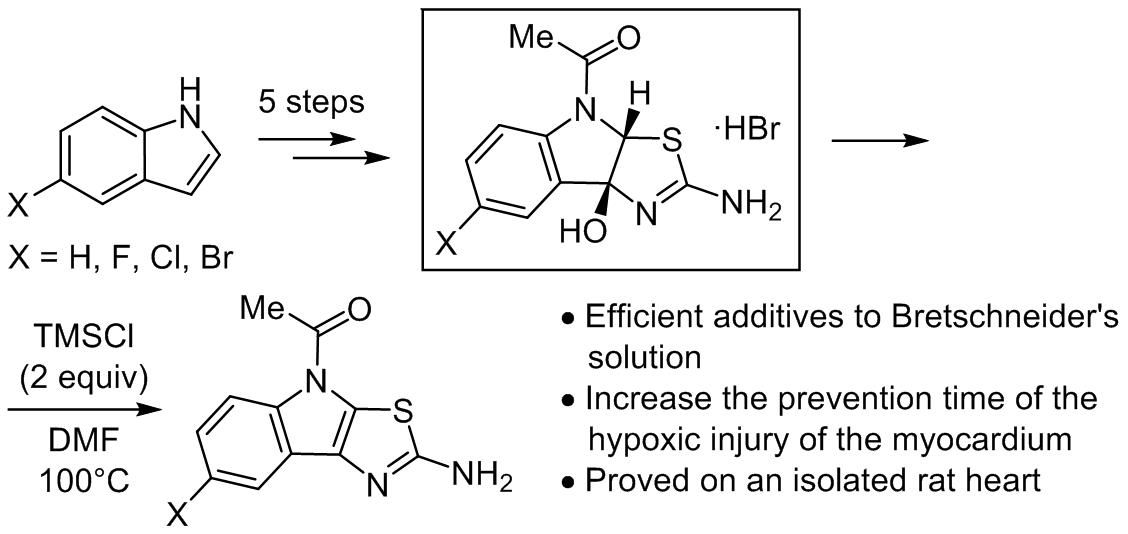
A set of 1-(2-amino-4H-thiazolo[5,4-b]indol-4-yl)ethan-1-one derivatives and their formally "hydrated" counterparts 1-(2-amino-8b-hydroxy-3a,8b-dihydro-4H-thiazolo[5,4-b]indol-4-yl)ethan-1-ones were synthesized and characterized by both spectral methods and single crystal X-ray diffraction studies. The obtained compounds as additives to Bretschneider's solution (trade name Custodiol) were tested on an isolated rat heart, as a classical model for the period of hypothermic ischemia. The experiments showed that adding the synthesized compounds to classical Bretschneider's solution increases the prevention time of the hypoxic injury of the myocardium by at least 45 min of exposure without oxygen supplements and significant histological changes. The "hydrated" forms showed much better results than the parent ones. Meanwhile, it was found that additive activity correlates with the substance's solubility in Custodiol. This data could be a milestone for the further medicinal chemistry project with the goal of developing a new generation of perfusion and flushing compositions for increasing the duration of open heart surgery.