IMPROVED SYNTHESIS OF TWO QUISQUALIC ACID ANALOGS CONTAINING HYDANTOIN AND IMIDAZOLIDINONE MOIETIES
Ключевые слова:
amino acids, cyclization, nitrogen heterocycles, reductive amination, SARS-CoV-2 main protease inhibitorsАннотация
In the light of recent progress in the development of SARS-CoV-2 main protease inhibitors, the synthesis of their key fragment,
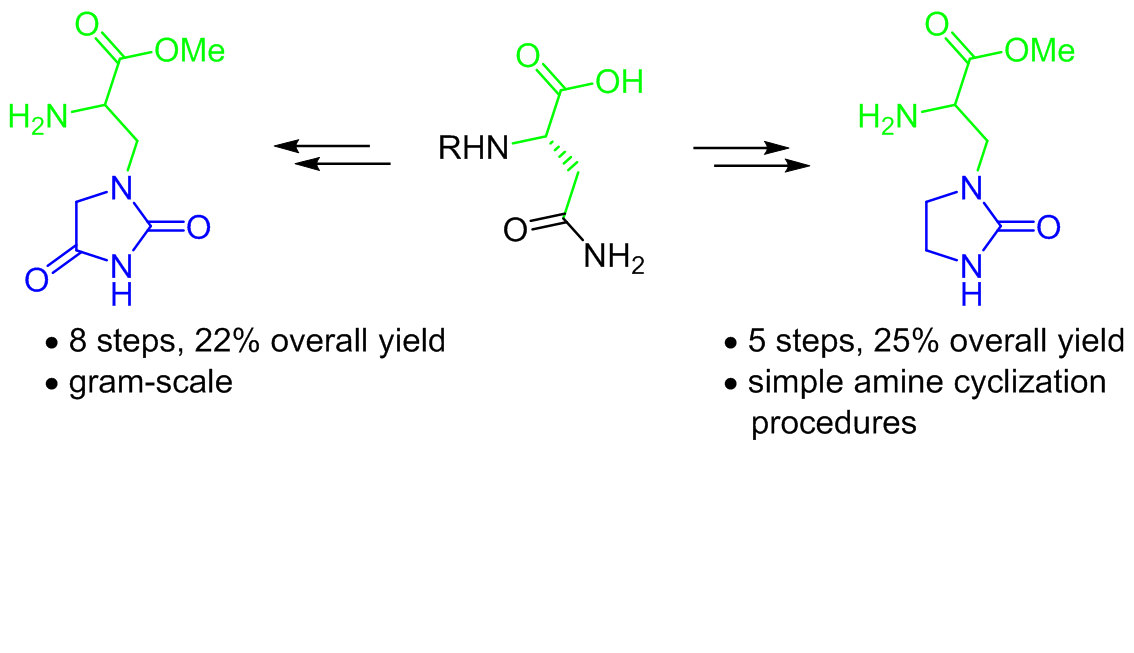
heterocyclic amino acids, is of great interest. Here, we report a method for the preparation of two new quisqualic acid analogs containing hydantoin and imidazolidinone moieties. The hydantoin analog was obtained using an amide ester cyclization, while the imidazolidinone unit was constructed by reductive amination and subsequent cyclization of a substituted ethylenediamine with carbonyldiimidazole. The presented approach provides the convergent synthesis of target analogs in 8 and 5 steps respectively.
Загрузки
Дополнительные файлы
Опубликован
2024-08-01
Выпуск
Раздел
Оригинальные статьи
