SYNTHESIS AND MODULATORY ACTIVITY OF 3-ACETYL-7-(BENZOFURAN- 5-CARBONYL)-1,5-DIMETHYL-3,7-DIAZABICYCLO[3.3.1]NONAN-9-ONE ON AMPA RECEPTORS
Ключевые слова:
3,7-diazabicyclo[3.3.1]nonanes, allosteric modulators, AMPA receptor, desymmetrization, monoacylation, neurodegenerative diseases, PAMs, patch clampАннотация
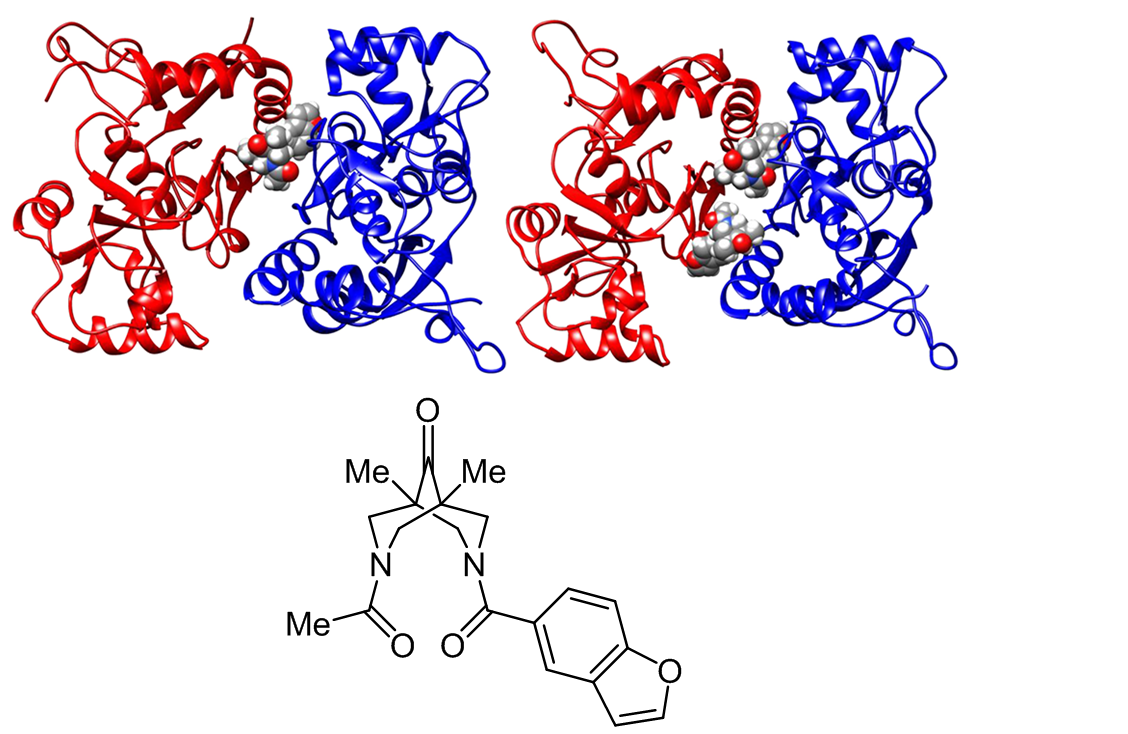
3-Acetyl-7-(benzofuran-5-carbonyl)-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonan-9-one belonging to a previously unknown class of unsymmetrical N,N'-diacyl-substituted 1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonan-9-ones has been synthesized. The key step in its synthesis involves a desymmetrization of the parent diamine by selective monoacetylation at a lower reaction temperature. In vitro assays by means of electrophysiological patch clamp technique revealed a high positive modulatory effect on the kainate-induced currents in Purkinje neurons in a wide concentration range from 10–11 to 10–6 M. Molecular dynamics simulations have confirmed the putative single and dual binding modes of the title compound in the positive allosteric modulator site of the AMPA (α-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid) glutamate receptors. These results indicate that it could potentially serve as a lead compound for the development of broad-spectrum drugs for prevention and treatment of neurodegenerative diseases.